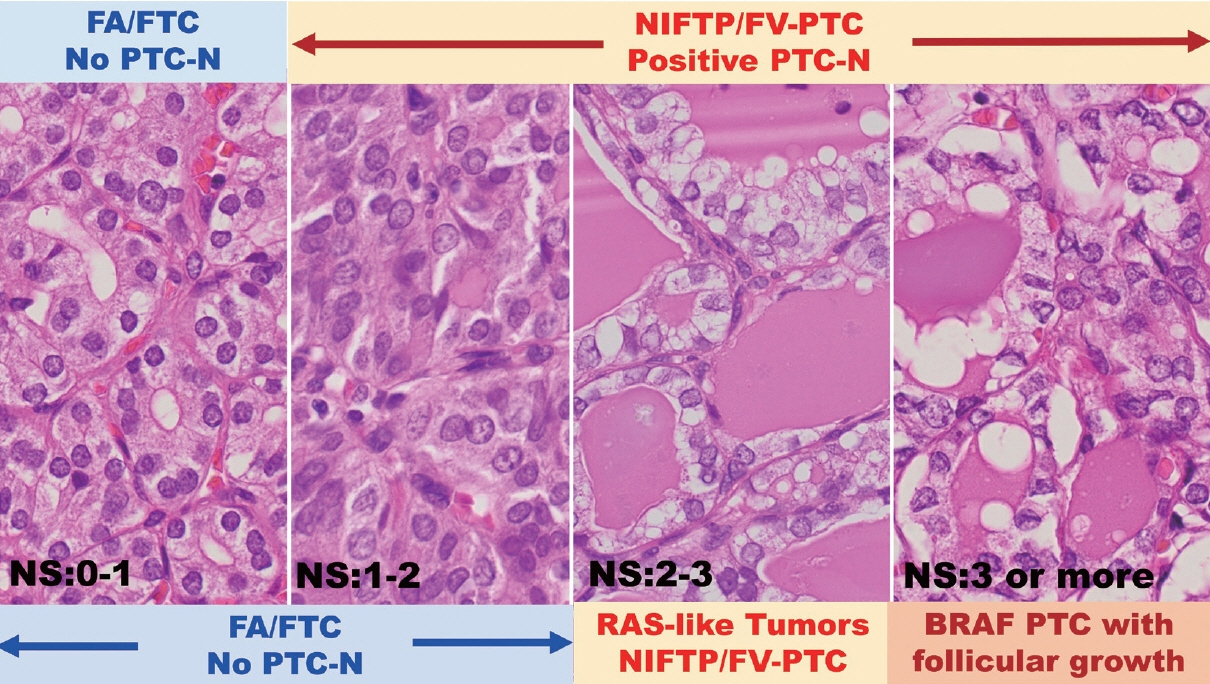
J Pathol Transl Med.
2023 Nov;57(6):289-304. 10.4132/jptm.2023.10.04.
The Asian Thyroid Working Group, from 2017 to 2023
- Affiliations
-
- 1Department of Pathology, Cancer Genome Center and Thyroid Disease Center, Izumi City General Hospital, Izumi, Osaka, Japan
- 2Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Pathology, Shanghai Sixth People’s Hospital, Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Japan
- 5Department of Pathology, Kameda Medical Center, Kamogawa, Chiba, Japan
- 6Department of Pathology, University of Iowa Hospitals and Clinics, Iowa City, IA, USA
- 7Special Task Force for Activating Research (STAR), Department of Pathology, Chulalongkorn University, Bangkok, Thailand
- 8Department of Cytology and Gynecological Pathology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- 9Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- KMID: 2547927
- DOI: http://doi.org/10.4132/jptm.2023.10.04
Abstract
- The Asian Thyroid Working Group was founded in 2017 at the 12th Asia Oceania Thyroid Association (AOTA) Congress in Busan, Korea. This group activity aims to characterize Asian thyroid nodule practice and establish strict diagnostic criteria for thyroid carcinomas, a reporting system for thyroid fine needle aspiration cytology without the aid of gene panel tests, and new clinical guidelines appropriate to conservative Asian thyroid nodule practice based on scientific evidence obtained from Asian patient cohorts. Asian thyroid nodule practice is usually designed for patient-centered clinical practice, which is based on the Hippocratic Oath, “First do not harm patients,” and an oriental filial piety “Do not harm one’s own body because it is a precious gift from parents,” which is remote from defensive medical practice in the West where physicians, including pathologists, suffer from severe malpractice climate. Furthermore, Asian practice emphasizes the importance of resource management in navigating the overdiagnosis of low-risk thyroid carcinomas. This article summarizes the Asian Thyroid Working Group activities in the past 7 years, from 2017 to 2023, highlighting the diversity of thyroid nodule practice between Asia and the West and the background reasons why Asian clinicians and pathologists modified Western systems significantly.
Keyword
Figure
Cited by 1 articles
-
Fine needle aspiration cytology diagnoses of follicular thyroid carcinoma: results from a multicenter study in Asia
Hee Young Na, Miyoko Higuchi, Shinya Satoh, Kaori Kameyama, Chan Kwon Jung, Su-Jin Shin, Shipra Agarwal, Jen-Fan Hang, Yun Zhu, Zhiyan Liu, Andrey Bychkov, Kennichi Kakudo, So Yeon Park
J Pathol Transl Med. 2024;58(6):331-340. doi: 10.4132/jptm.2024.10.12.
Reference
-
References
1. Ali SZ, Vanderlaan P. The Bethesda System for Reporting Thyroid Cytopathology. 3rd ed. New York: Springer;2023.2. WHO Classification of Tumours Editorial Board. WHO classification of tumours of endocrine organs. 5th ed. Lyon: IARC Press;2022.3. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.
Article4. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.5. Kakudo K, Katoh R, Sakamoto A, et al. Thyroid gland: international case conference. Endocr Pathol. 2002; 13:131–4.
Article6. Hirokawa M, Carney JA, Goellner JR, et al. Observer variation of encapsulated follicular lesions of the thyroid gland. Am J Surg Pathol. 2002; 26:1508–14.
Article7. Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004; 28:1336–40.
Article8. Liu Z, Zhou G, Nakamura M, et al. Encapsulated follicular thyroid tumor with equivocal nuclear changes, so-called well-differentiated tumor of uncertain malignant potential: a morphological, immunohistochemical, and molecular appraisal. Cancer Sci. 2011; 102:288–94.
Article9. Kakudo K, Kameyama K, Miyauchi A, Nakamura H. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J. 2014; 61:539–52.
Article10. Kakudo K, Kameyama K, Hirokawa M, Katoh R, Nakamura H. Subclassification of follicular neoplasms recommended by the Japan thyroid association reporting system of thyroid cytology. Int J Endocrinol. 2015; 2015:938305.
Article11. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice: active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology. 2017; 28:455–66.
Article12. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Thyroid. 2017; 27:983–4.
Article13. Vuong HG, Ngo HT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol. 2020; 128:238–49.
Article14. Kakudo K. Asian and Western practice in thyroid pathology: similarities and differences. Gland Surg. 2020; 9:1614–27.
Article15. Hirokawa M, Auger M, Jung CK, Callegari FM. Thyroid FNA cytology: the Eastern versus Western perspectives. Cancer Cytopathol. 2023; 131:415–20.
Article16. Kakudo K, Liu Z, Jung CK, Lai CR. Lessons learned from the diversity of thyroid nodule practice. Cancer Cytopathol. 2023 Jun 6 [Epub]. https://doi.org/10.1002/cncy.22728.
Article17. Bychkov A, Schubert M. Constant demand, patchy supply. Pathologist. 2023; 88:18–27.18. Reisch LM, Carney PA, Oster NV, et al. Medical malpractice concerns and defensive medicine: a nationwide survey of breast pathologists. Am J Clin Pathol. 2015; 144:916–22.19. Labarge B, Walter V, Lengerich EJ, et al. Evidence of a positive association between malpractice climate and thyroid cancer incidence in the United States. PLoS One. 2018; 13:e0199862.
Article20. Kakudo K, Bychkov A, Abelardo A, Keelawat S, Kumarasinghe P. Malpractice climate is a key difference in thyroid pathology practice between North America and the rest of the world. Arch Pathol Lab Med. 2019; 143:1171.
Article21. Renshaw AA, Gould EW. Why there is the tendency to “overdiagnose” the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002; 117:19–21.
Article22. Tallini G, Tuttle RM, Ghossein RA. The history of the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2017; 102:15–22.
Article23. Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016; 2:1023–9.
Article24. Lee YS, Nam KH, Chung WY, et al. Practical management of well differentiated thyroid carcinoma in Korea. Endocr J. 2008; 55:1015–24.
Article25. Takami H, Ito Y, Okamoto T, Onoda N, Noguchi H, Yoshida A. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg. 2014; 38:2002–10.
Article26. Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020; 67:669–717.
Article27. Welch HG, Doherty GM. Saving thyroids: overtreatment of small papillary cancers. N Engl J Med. 2018; 379:310–2.
Article28. Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003; 13:381–7.
Article29. Castro MR, Morris JC, Ryder M, Brito JP, Hay ID. Most patients with a small papillary thyroid carcinoma enjoy an excellent prognosis and may be managed with minimally invasive therapy or active surveillance. Cancer. 2015; 121:3364–5.
Article30. Sugitani I, Ito Y, Miyauchi A, Imai T, Suzuki S. Active surveillance versus immediate surgery: questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid. 2019; 29:1563–71.
Article31. Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid. 2021; 31:183–92.
Article32. Ghossein R, Barletta JA, Bullock M, et al. Data set for reporting carcinoma of the thyroid: recommendations from the International Collaboration on Cancer Reporting. Hum Pathol. 2021; 110:62–72.
Article33. Shindo H, Kakudo K, Inomata K, et al. Additional tissue sampling trials did not change our thyroid practice. Cancers (Basel). 2021; 13:1270.
Article34. Kakudo K, Li Y, Taniguchi E, Liu Z. Follicular neoplasms in the 4th edition WHO classification of endocrine organs. J Basic Clin Med. 2018; 7:10–9.35. Jung CK, Bychkov A, Song DE, et al. Molecular correlates and nuclear features of encapsulated follicular-patterned thyroid neoplasms. Endocrinol Metab (Seoul). 2021; 36:123–33.
Article36. Chan J. Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2002; 117:16–8.
Article37. Piana S, Frasoldati A, Di Felice E, Gardini G, Tallini G, Rosai J. Encapsulated well-differentiated follicular-patterned thyroid carcinomas do not play a significant role in the fatality rates from thyroid carcinoma. Am J Surg Pathol. 2010; 34:868–72.
Article38. Chetty R. A proposal for the classification of follicular-patterned neoplasms of the thyroid gland. Am J Surg Pathol. 2011; 35:313.
Article39. Goffredo P, Cheung K, Roman SA, Sosa JA. Can minimally invasive follicular thyroid cancer be approached as a benign lesion?: a population-level analysis of survival among 1,200 patients. Ann Surg Oncol. 2013; 20:767–72.
Article40. Kakudo K. Different threshold of malignancy for RAS-like thyroid tumors causes significant differences in thyroid nodule practice. Cancers (Basel). 2022; 14:812.
Article41. Kakudo K, El-Naggar AK, Hodak SP, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) in thyroid tumor classification. Pathol Int. 2018; 68:327–33.
Article42. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC Press;2017.43. Bai Y, Kakudo K, Jung CK. Updates in the pathologic classification of thyroid neoplasms: a review of the World Health Organization classification. Endocrinol Metab (Seoul). 2020; 35:696–715.
Article44. Bychkov A, Keelawat S, Agarwal S, et al. Impact of non-invasive follicular thyroid neoplasm with papillary-like nuclear features on the Bethesda system for reporting thyroid cytopathology: a multiinstitutional study in five Asian countries. Pathology. 2018; 50:411–7.
Article45. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol. 2018; 29:276–88.
Article46. Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int. 2019; 69:202–10.
Article47. Vuong HG, Tran TT, Bychkov A, et al. Clinical impact of non-Invasive follicular thyroid neoplasm with papillary-like nuclear features on the risk of malignancy in the Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis of 14,153 resected thyroid nodules. Endocr Pract. 2019; 25:491–502.
Article48. Rana C, Vuong HG, Nguyen TQ, et al. The incidence of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a meta-analysis assessing worldwide impact of the reclassification. Thyroid. 2021; 31:1502–13.
Article49. Bychkov A, Kakudo K, Hong S. Current practices of thyroid fineneedle aspiration in Asia: a missing voice. J Pathol Transl Med. 2017; 51:517–20.
Article50. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med. 2017; 51:571–8.
Article51. Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod Pathol. 2016; 29:698–707.
Article52. Kakudo K. Thyroid FNA cytology: differential diagnoses and pitfalls. 3rd ed. Singapore: Springer;2023.53. Hirokawa M, Suzuki A, Hashimoto Y, et al. Prevalence and diagnostic challenges of thyroid lymphoma: a multi-institutional study in non-Western countries. Endocr J. 2020; 67:1085–91.
Article54. Agarwal S, Bychkov A, Jung CK, et al. The prevalence and surgical outcomes of Hurthle cell lesions in FNAs of the thyroid: a multiinstitutional study in 6 Asian countries. Cancer Cytopathol. 2019; 127:181–91.55. Liu CY, Bychkov A, Agarwal S, et al. Cytologic diagnosis of medullary thyroid carcinoma in the Asia-Pacific region. Diagn Cytopathol. 2021; 49:60–9.56. Liu CY, Chen CC, Bychkov A, et al. Constitutive cytomorphologic features of medullary thyroid carcinoma using different staining methods. Diagnostics (Basel). 2021; 11:1396.
Article57. Jung CK, Agarwal S, Hang JF, Lim DJ, Bychkov A, Mete O. Update on C-cell neuroendocrine neoplasm: prognostic and predictive histopathologic and molecular features of medullary thyroid carcinoma. Endocr Pathol. 2023; 34:1–22.
Article58. Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms: an observer variation study. Endocr Pathol. 2020; 31:132–40.
Article59. Lai WA, Hang JF, Liu CY, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multiinstitutional study of 182 cases using the monoclonal antibody MRQ-50. Virchows Arch. 2020; 476:431–7.
Article60. Ngo TN, Le TTB, Le T, et al. Primary versus secondary anaplastic thyroid carcinoma: perspectives from multi-institutional and population-level data. Endocr Pathol. 2021; 32:489–500.
Article61. Ohba K, Mitsutake N, Matsuse M, et al. Encapsulated papillary thyroid tumor with delicate nuclear changes and a KRAS mutation as a possible novel subtype of borderline tumor. J Pathol Transl Med. 2019; 53:136–41.
Article62. Jung CK, Park SY, Kim JH, Kakudo K. New insights into classification and risk stratification of encapsulated thyroid tumors with a predominantly papillary architecture. J Pathol Transl Med. 2020; 54:197–203.
Article63. Giani C, Torregrossa L, Ramone T, et al. Whole tumor capsule is prognostic of very good outcome in the classical variant of papillary thyroid cancer. J Clin Endocrinol Metab. 2021; 106:e4072–83.
Article64. Nguyen TP, Truong VT, Kakudo K, Vuong HG. The diversities in thyroid cytopathology practices among Asian countries using the Bethesda System for Reporting Thyroid Cytopathology. Gland Surg. 2020; 9:1735–46.
Article65. Vuong HG, Nguyen TP, Hassell LA, Jung CK. Diagnostic performances of the Afirma Gene Sequencing Classifier in comparison with the Gene Expression Classifier: a meta-analysis. Cancer Cytopathol. 2021; 129:182–9.
Article66. Ngo HT, Nguyen TP, Vu TH, et al. Impact of molecular testing on the management of indeterminate thyroid nodules among Western and Asian countries: a systematic review and meta-analysis. Endocr Pathol. 2021; 32:269–79.
Article67. Vuong HG, Suzuki A, Na HY, et al. Application of the Bethesda System for Reporting Thyroid Cytopathology in the pediatric population. Am J Clin Pathol. 2021; 155:680–9.
Article68. Vuong HG, Chung DG, Ngo LM, et al. The use of the Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: a meta-analysis. Thyroid. 2021; 31:1203–11.
Article69. Vuong HG, Jung CK, Kakudo K, Bychkov A. Response to Cherella et al. re: “the use of the Bethesda System for Reporting Thyroid Cytopathology in pediatric thyroid nodules: a meta-analysis”. Thyroid. 2021; 31:1442–4.
Article70. Wang YH, Bychkov A, Chakrabarti I, et al. Impact of the COVID-19 pandemic on cytology practice: an international survey in the Asia-Pacific region. Cancer Cytopathol. 2020; 128:895–904.
Article71. Vuong HG, Le HT, Le TT, Le T, Hassell L, Kakudo K. Clinicopathological significance of major fusion oncogenes in papillary thyroid carcinoma: an individual patient data meta-analysis. Pathol Res Pract. 2022; 240:154180.
Article72. Vuong HG, Le MK, Hassell L, Kondo T, Kakudo K. The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck. 2022; 44:926–32.
Article73. Vuong HG, Nguyen TP, Ngo HT, Hassell L, Kakudo K. Malignant thyroid teratoma: an integrated analysis of case series/case reports. Endocr Relat Cancer. 2021; 28:495–503.
Article74. Le HT, Nguyen TP, Hirokawa M, et al. Primary thyroid mucoepidermoid carcinoma (MEC) is clinically, prognostically, and molecularly different from sclerosing MEC with eosinophilia: a multicenter and integrated study. Endocr Pathol. 2023; 34:100–11.
Article75. LiVolsi VA, Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid. 1994; 4:233–6.
Article76. Cipriani NA, Nagar S, Kaplan SP, et al. Follicular thyroid carcinoma: how have histologic diagnoses changed in the last half-century and what are the prognostic implications? Thyroid. 2015; 25:1209–16.
Article77. Kakudo K. Non-invasive encapsulated/well-circumscribed follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and precursor thyroid tumors. J Basic Clin Med. 2017; 6:1–2.78. Kakudo K. Unsettled issues in non-invasive encapsulated/wellcircumscribed follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) and precursor thyroid tumors. J Basic Clin Med. 2017; 6:3–7.79. LiVolsi VA, Baloch ZW. Coming to terms with diagnosis “non-invasive follicular neoplasm with papillary like nuclear features (NIFTP)’: practice changer in endocrine pathology. J Basic Clin Med. 2017; 6:8–13.80. Kakudo K, Liu Z, Satoh S, Higuchi M, Hirokawa M. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): diagnosis and differential diagnoses. J Basic Clin Med. 2017; 6:14–21.81. Liu Z, Song Y, Han B, Zhang X, Su P, Cui X. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features and the practice in Qilu Hospital of Shandong University, China. J Basic Clin Med. 2017; 6:22–5.82. Jung CK, Kim C. Effect of lowering the diagnostic threshold for encapsulated follicular variant of papillary thyroid carcinoma on the prevalence of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: a single-institution experience in Korea. J Basic Clin Med. 2017; 6:26–8.83. Pusztaszeri MP, Triponez F, Meyer P, Sadowski SM. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): report of an institutional experience with 86 cases. J Basic Clin Med. 2017; 6:29–35.84. Rossi ED. NIFTP diagnosis: roses and thorns for cytopathologists and histopathologists. J Basic Clin Med. 2017; 6:36–7.85. Canberk S, Baloch ZW, Ince U, Schmitt F. Diagnosis of non-invasive follicular tumor with papillary-like nuclear features (NIFTP): a practice changer for thyroid fine-needle aspiration interpretation. J Basic Clin Med. 2017; 6:38–43.86. Maletta F, Volante M, Papotti M. Experience on NIFTP cytology, with a mini meta-analysis of the literature. J Basic Clin Med. 2017; 6:44–50.87. Ng D, Can NT, Ma ZV, van Zante A, Ljung BM, Khanafshar E. Cytomorphologic features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a comparison with infiltrative follicular variant of papillary thyroid carcinoma. J Basic Clin Med. 2017; 1:51–6.88. Saglietti C, Bongiovanni M. The value of cytological examination in the diagnosis of noninvasive thyroid neoplasm with papillarylike nuclear features (NIFTP). J Basic Clin Med. 2017; 6:57–60.89. Wu HH. The impact of NIFTP on FNA cytology: can we still diagnose papillary thyroid carcinoma? J Basic Clin Med. 2017; 6:61–2.90. Yang GC. Ultrasound is cytopathologist’s best friend in the era of noninvasive follicular thyroid neoplasm with papillary-like nuclear features. J Basic Clin Med. 2017; 6:65–7.91. Poller DN, Johnson SJ, Stephenson TJ. Diagnosis of NIFTP in the UK. J Basic Clin Med. 2017; 6:63–4.92. Cha YJ, Pyo JY, Hong S, et al. Thyroid fine-needle aspiration cytology practice in Korea. J Pathol Transl Med. 2017; 51:521–7.
Article93. Liu Z, Liu D, Ma B, et al. History and practice of thyroid fine-needle aspiration in China, based on retrospective study of the practice in Shandong University Qilu Hospital. J Pathol Transl Med. 2017; 51:528–32.
Article94. Agarwal S, Jain D. Thyroid cytology in India: contemporary review and meta-analysis. J Pathol Transl Med. 2017; 51:533–47.
Article95. Satoh S, Yamashita H, Kakudo K. Thyroid cytology: the Japanese system and experience at Yamashita Thyroid Hospital. J Pathol Transl Med. 2017; 51:548–54.
Article96. Abelardo AD. Thyroid fine-needle aspiration practice in the Philippines. J Pathol Transl Med. 2017; 51:555–9.
Article97. Hang JF, Hsu CY, Lai CR. Thyroid fine-needle aspiration in Taiwan: the history and current practice. J Pathol Transl Med. 2017; 51:560–4.
Article98. Keelawat S, Rangdaeng S, Koonmee S, Jitpasutham T, Bychkov A. Current status of thyroid fine-needle aspiration practice in Thailand. J Pathol Transl Med. 2017; 51:565–70.
Article99. Ohori NP. Molecular testing and thyroid nodule management in North America. Gland Surg. 2020; 9:1628–38.
Article100. Kumarasinghe MP. Standardisation of thyroid cytology terminology and practice: are modifications necessary?: a narrative review. Gland Surg. 2020; 9:1639–47.
Article101. Poller DN. Litigation in thyroid cytology and histopathology in England: a very brief overview. Gland Surg. 2020; 9:1648–52.
Article102. Hirokawa M, Suzuki A, Higuchi M, et al. The Japanese reporting system for thyroid aspiration cytology 2019 (JRSTAC2019). Gland Surg. 2020; 9:1653–62.
Article103. Ito Y, Miyauchi A. Active surveillance of low-risk papillary thyroid microcarcinomas. Gland Surg. 2020; 9:1663–73.
Article104. Zhu Y, Wu H, Huang B, Shen X, Cai G, Gu X. BRAF(V600E) mutation combined with American College of Radiology thyroid imaging report and data system significantly changes surgical resection rate and risk of malignancy in thyroid cytopathology practice. Gland Surg. 2020; 9:1674–84.
Article105. Pusztaszeri MP, Tamilia M, Payne RJ. Active surveillance for lowrisk small papillary thyroid cancer in North American countries: past, present and future (bridging the gap between North American and Asian practices). Gland Surg. 2020; 9:1685–97.
Article106. Okamoto T, Omi Y, Yoshida Y, Horiuchi K, Abe K. Radioactive iodine treatment of papillary thyroid carcinoma in Japan. Gland Surg. 2020; 9:1698–707.
Article107. Michael CW, Kameyama K, Kitagawa W, Azar N. Rapid on-site evaluation (ROSE) for fine needle aspiration of thyroid: benefits, challenges and innovative solutions. Gland Surg. 2020; 9:1708–15.
Article108. Liu Z, Sui S, Su P, et al. The effect of implementing pre-surgical ultrasound-guided fine-needle aspiration biopsy on thyroid surgery, a 6-year interrupted time series analysis in Qilu Hospital of Shandong University. Gland Surg. 2020; 9:1716–23.
Article109. Canberk S. Precursor and borderline lesions of the thyroid (indolent lesions of epithelial origin): from theory to practice. Gland Surg. 2020; 9:1724–34.
Article110. Oo ZP, Hlaing AM, Kyi KC, Fukuoka J, Bychkov A. An overview of thyroid fine-needle aspiration practice in Myanmar. Gland Surg. 2020; 9:1747–53.
Article111. Tangnuntachai N, Rangdaeng S, Koonmee S, Tangjaturonrasme N, Keelawat S. Pathological practice and management of thyroid nodules: a Thai perspective. Gland Surg. 2020; 9:1754–63.
Article112. Ooi LY, Nga ME. Atypia of undetermined significance/follicular lesion of undetermined significance: Asian vs. non-Asian practice, and the Singapore experience. Gland Surg. 2020; 9:1764–87.
Article113. Abelardo AD, Sotalbo KCJ. Clinical management of thyroid aspirates diagnosed as atypia of undetermined significance in the Philippines. Gland Surg. 2020; 9:1788–96.
Article114. Guleria P, Mani K, Agarwal S. Indian experience of AUS/FLUS diagnosis: is it different from rest of Asia and the West?: A systematic review and meta-analysis. Gland Surg. 2020; 9:1797–812.
Article115. Odate T, Oishi N, Vuong HG, Mochizuki K, Kondo T. Genetic differences in follicular thyroid carcinoma between Asian and Western countries: a systematic review. Gland Surg. 2020; 9:1813–26.
Article116. Nakra T, Jain D, Agarwal S. Thyroid lymphoproliferative lesions in Asia. Gland Surg. 2020; 9:1827–37.
Article117. Li Y, Inomata K, Nishihara E, Kakudo K. IgG4 thyroiditis in the Asian population. Gland Surg. 2020; 9:1838–46.
Article118. Bai Y, Niu D, Yao Q, Lin D, Kakudo K. Updates in the advances of sporadic medullary thyroid carcinoma: from the molecules to the clinic. Gland Surg. 2020; 9:1847–56.
Article119. Jung CK, Lee S, Bae JS, Lim DJ. Late-onset distant metastases confer poor prognosis in patients with well-differentiated thyroid cancer. Gland Surg. 2020; 9:1857–66.
Article120. Choden S, Keelawat S, Jung CK, Bychkov A. An affordable immunohistochemical approach to estimate the prevalence of BRAF (V600E) in large cohort studies: establishing the baseline rate of BRAF mutation in an institutional series of papillary thyroid carcinoma from Thailand. Gland Surg. 2020; 9:1867–77.
Article121. Rashid FA, Munkhdelger J, Fukuoka J, Bychkov A. Prevalence of BRAF(V600E) mutation in Asian series of papillary thyroid carcinoma: a contemporary systematic review. Gland Surg. 2020; 9:1878–900.
Article122. Kakudo K, Pusztaszeri M, Bongiovanni M. Factors impacting thyroid fine needle aspiration cytology and algorithm for cytological diagnosis in the Japanese system for reporting FNA cytology. Kakudo K, editor. Thyroid FNA cytology, differential diagnoses and pitfalls. 2nd ed. Singapore: Springer Singapore;2019. p. 19–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Use of Fine-Needle Aspiration (FNA) Cytology in Patients with Thyroid Nodules in Asia: A Brief Overview of Studies from the Working Group of Asian Thyroid FNA Cytology
- Differentiated Thyroid Cancer in Asians
- The Third Asian Radiology Forum: Screening Programs in Asia
- Relationship Between Long Working Hours and Metabolic Syndrome Among Korean Workers
- RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology