Obstet Gynecol Sci.
2023 Nov;66(6):537-544. 10.5468/ogs.23124.
A simplified two-marker immunohistochemistry strategy for Lynch syndrome screening in endometrial cancer patients
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Galilee Medical Center, Nahariya, Azrieli Faculty of Medicine, Bar Ilan University, Safed, Israel
- 2Department of Obstetrics and Gynecology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 4Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 5Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2547855
- DOI: http://doi.org/10.5468/ogs.23124
Abstract
Objective
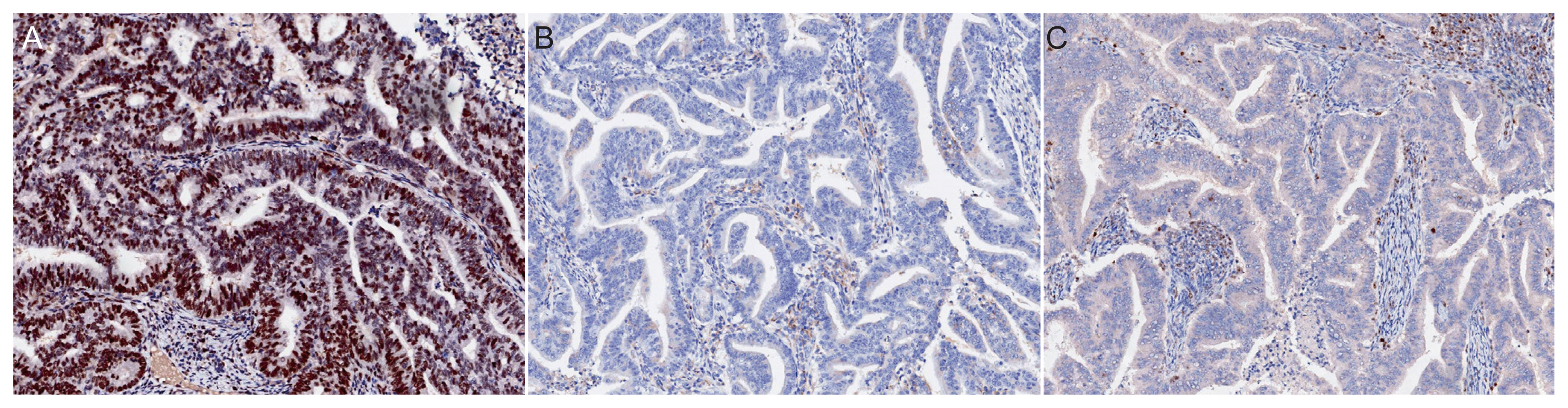
To examine the efficacy of MSH6 and PMS2 immunohistochemistry (IHC) as a screening method for Lynch syndrome in endometrial cancer patients.
Methods
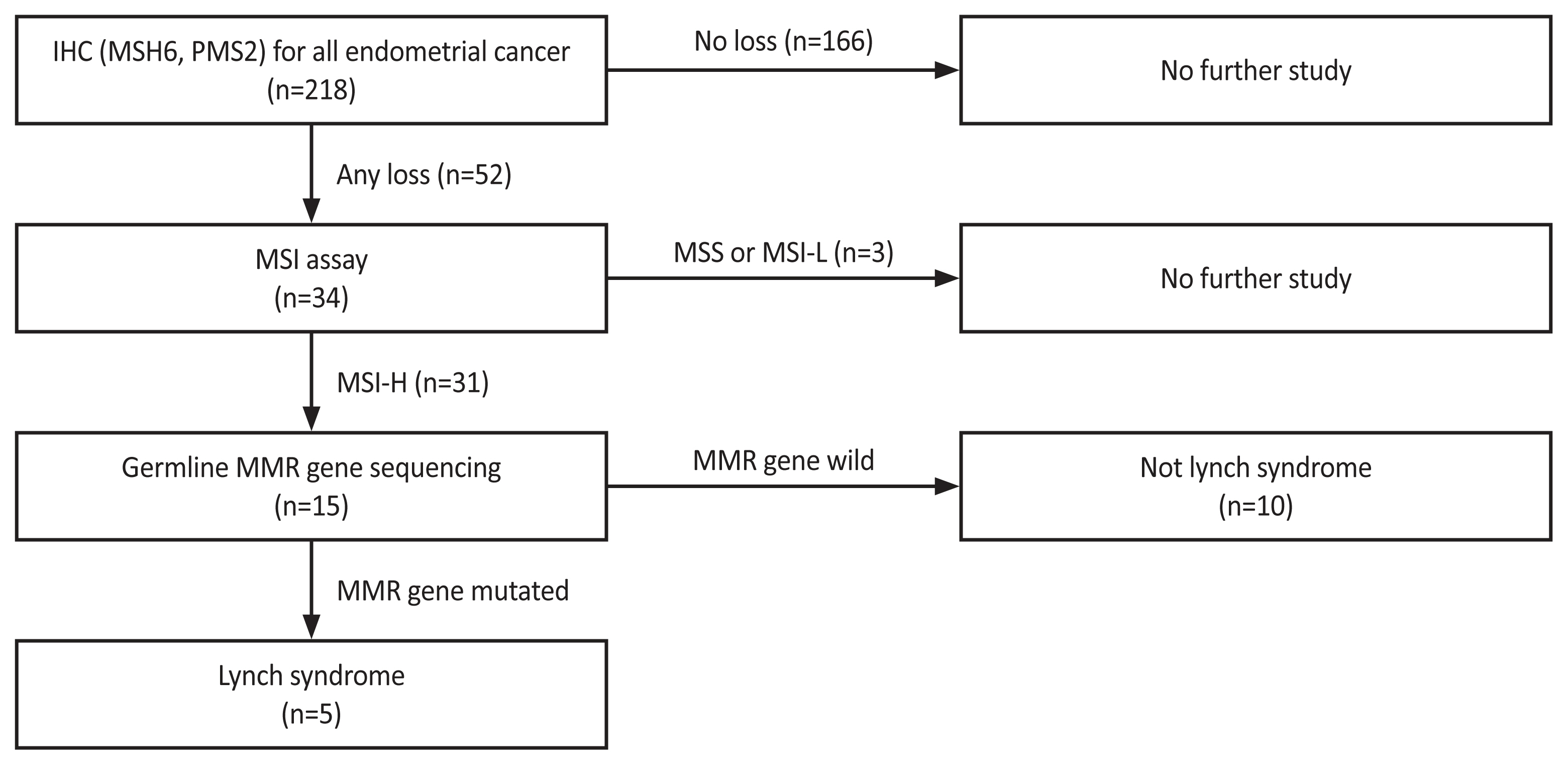
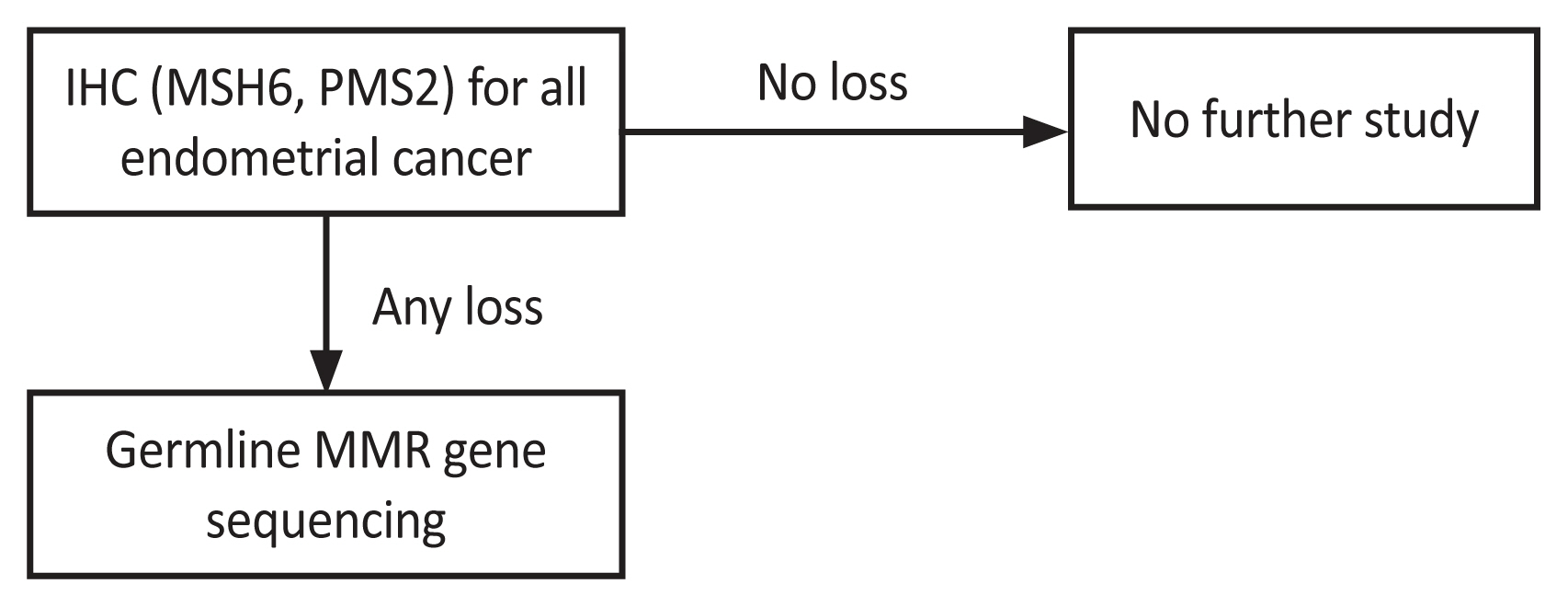
Through multidisciplinary discussions, an institutional MSH6 and PMS2 IHC-initiated cascade test (MSH6, PMS2 IHC→microsatellite instability [MSI] assay→germline mismatch repair [MMR] gene sequencing) was developed to screen for Lynch syndrome in endometrial cancer patients. Testing was performed on a consecutive cohort of 218 newly diagnosed endometrial cancer patients who underwent surgery at a tertiary hospital in the Republic of Korea between August 2018 and December 2020. The number of MMR deficiencies (MSH6 or PMS2 loss in IHC) and results of subsequent tests (MSI assay and germline MMR gene sequencing) were examined.
Results
MMR deficiency was detected in 52 of the 218 patients (24.0%). Among these 52 patients, 34 (65.0%) underwent MSI testing, of which 31 (91.0%) exhibited high MSI. Of the 31 patients with MSI-high status, 15 (48.0%) underwent germline MMR gene sequencing. Subsequently, Lynch syndrome was diagnosed in five patients (33.0%).
Conclusion
Lynch syndrome screening using MSH6 and PMS2 IHC-initiated cascade testing is a viable strategy in the management of endometrial cancer. A simplified strategy (MSH6 and PMS2 IHC→germline MMR gene sequencing) was proposed because most women with MMR deficiencies exhibited high MSI.
Figure
Reference
-
References
1. Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015; 15:181–94.
Article2. Ahadova A, Gallon R, Gebert J, Ballhausen A, Endris V, Kirchner M, et al. Three molecular pathways model colorectal carcinogenesis in Lynch syndrome. Int J Cancer. 2018; 143:139–50.3. Barrow E, Robinson L, Alduaij W, Shenton A, Clancy T, Lalloo F, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet. 2009; 75:141–9.
Article4. Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2017; 66:464–72.
Article5. Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a “sentinel cancer” for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005; 105:569–74.
Article6. Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016; 15:423–7.
Article7. Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. Gastroenterology. 1999; 116:1453–6.
Article8. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–8.9. Kalloger SE, Allo G, Mulligan AM, Pollett A, Aronson M, Gallinger S, et al. Use of mismatch repair immunohistochemistry and microsatellite instability testing: Exploring Canadian practices. Am J Surg Pathol. 2012; 36:560–9.10. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005; 352:1851–60.
Article11. Cohen SA. Current Lynch syndrome tumor screening practices: a survey of genetic counselors. J Genet Couns. 2014; 23:38–47.
Article12. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine neoplasms, version 1.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:170–99.
Article13. Martin SA, Lord CJ, Ashworth A. Therapeutic targeting of the DNA mismatch repair pathway. Clin Cancer Res. 2010; 16:5107–13.
Article14. Adar T, Rodgers LH, Shannon KM, Yoshida M, Ma T, Mattia A, et al. Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer. 2018; 124:3145–53.
Article15. Chao X, Li L, Wu M, Ma S, Tan X, Zhong S, et al. Comparison of screening strategies for Lynch syndrome in patients with newly diagnosed endometrial cancer: a prospective cohort study in China. Cancer Commun (Lond). 2019; 39:42.
Article16. Stelloo E, Jansen AML, Osse EM, Nout RA, Creutzberg CL, Ruano D, et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2017; 28:96–102.
Article17. Kim G, Lee SK, Suh DH, Kim K, No JH, Kim YB, et al. Clinical evaluation of a droplet digital PCR assay for detecting POLE mutations and molecular classification of endometrial cancer. J Gynecol Oncol. 2022; 33:e15.18. Yuan L, Chi Y, Chen W, Chen X, Wei P, Sheng W, et al. Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int J Clin Exp Med. 2015; 8:20988–1000.19. Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology group study. J Clin Oncol. 2015; 33:4301–8.20. Goverde A, Spaander MC, van Doorn HC, Dubbink HJ, van den Ouweland AM, Tops CM, et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70years of age. Gynecol Oncol. 2016; 143:453–9.21. Cohen SA, Leininger A. The genetic basis of Lynch syndrome and its implications for clinical practice and risk management. Appl Clin Genet. 2014; 7:147–58.
Article22. Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014; 38:1501–9.
Article23. Tranø G, Sjursen W, Wasmuth HH, Hofsli E, Vatten LJ. Performance of clinical guidelines compared with molecular tumour screening methods in identifying possible Lynch syndrome among colorectal cancer patients: a Norwegian population-based study. Br J Cancer. 2010; 102:482–8.
Article24. Johnatty SE, Tan YY, Buchanan DD, Bowman M, Walters RJ, Obermair A, et al. Family history of cancer predicts endometrial cancer risk independently of Lynch syndrome: implications for genetic counselling. Gynecol Oncol. 2017; 147:381–7.
Article25. Talseth-Palmer BA, Bauer DC, Sjursen W, Evans TJ, McPhillips M, Proietto A, et al. Targeted next-generation sequencing of 22 mismatch repair genes identifies Lynch syndrome families. Cancer Med. 206. 5:929–41.
Article26. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endometrial cancer six years after colon cancer in Lynch syndrome: Single institution case in Korea
- Hereditary Colon Cancer: Lynch Syndrome
- One case of endometrial cancer occurrence: Over 10 years after colon cancer in Lynch family
- A Case of Perimenopausal Endometrial Cancer in a Woman with MSH2 Germline Mutation
- Screening for Lynch syndrome using risk assessment criteria in patients with ovarian cancer