Cancer Res Treat.
2023 Oct;55(4):1363-1368. 10.4143/crt.2023.371.
Long-term Complete Remission of Decitabine-Primed Tandem CD19/CD22 CAR-T Therapy with PD-1 and BTK Inhibitors Maintenance in a Refractory Primary Central Nervous System Lymphoma Patient
- Affiliations
-
- 1College of Pharmaceutical Sciences Soochow University, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2National Clinical Research Center for Hematologic Diseases, Jiangsu Institute of Hematology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 3Institute of Blood and Marrow Transplantation, Collaborative Innovation Center of Hematology, Soochow University, Suzhou, China
- 4Department Of Hematology & Oncology, Wuxi Taihu Lake Hospital, Wuxi, China
- 5Department of Radiology, People’s Hospital of Binhai County, Yancheng, China
- 6Department of Pharmacy, The First Affiliated Hospital of Soochow University, Suzhou, China
- 7School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, China
- 8Shanghai Unicar-Therapy Bio-medicine Technology Co., Ltd., Shanghai, China
- KMID: 2547810
- DOI: http://doi.org/10.4143/crt.2023.371
Abstract
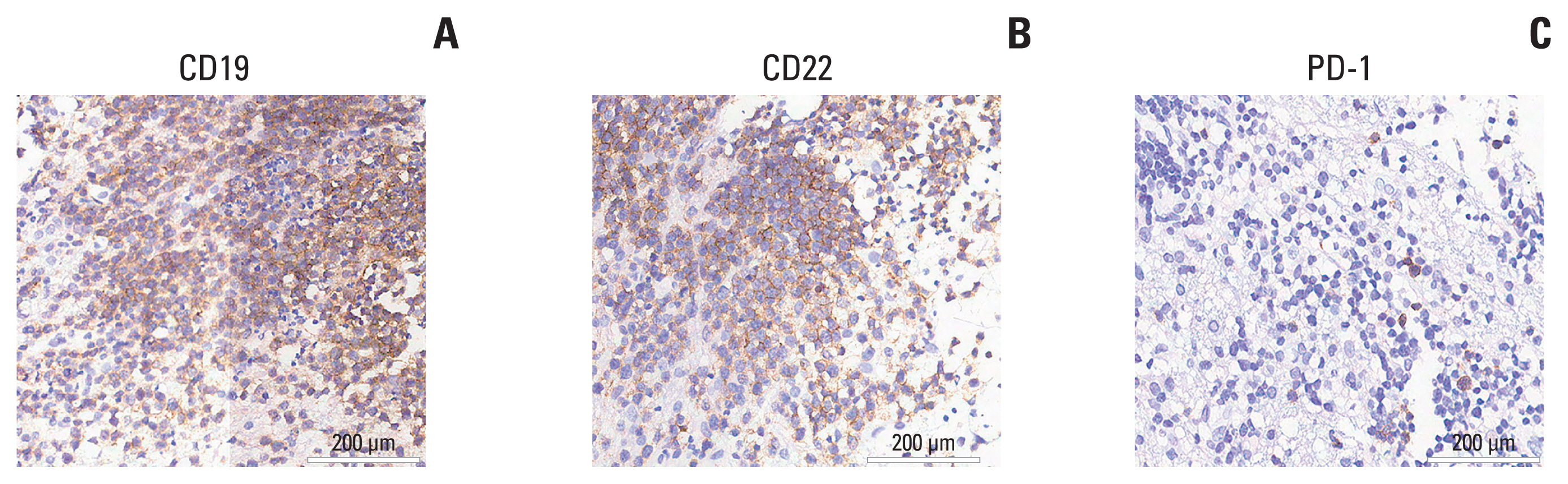
- Primary central nervous system lymphoma (PCNSL) is a rare and aggressive non-Hodgkin’s lymphoma that affects the brain, eyes, cerebrospinal fluid, or spinal cord without systemic involvement. The outcome of patients with PCNSL is worse compared to patients with systemic diffuse large B-cell lymphoma. Given potential mortality associated with severe immune effector cell-associated neurotoxicity syndrome (ICANS), patients with PCNSL have been excluded from most clinical trials involving chimeric antigen receptor T-cell (CAR-T) therapy initially. Here, we report for the first time to apply decitabine-primed tandem CD19/CD22 dual-targeted CAR-T therapy with programmed cell death-1 (PD-1) and Bruton’s tyrosine kinase (BTK) inhibitors maintenance in one patient with multiline-resistant refractory PCNSL and the patient has maintained complete remission (CR) for a 35-month follow-up period. This case represents the first successful treatment of multiline resistant refractory PCNSL with long-term CR and without inducing ICANS under tandem CD19/CD22 bispecific CAR-T therapy followed by maintenance therapy with PD-1 and BTK inhibitors. This study shows tremendous potential in the treatment of PCNSL and offers a look toward ongoing clinical studies.
Keyword
Figure
Reference
-
References
1. Yuan Y, Ding T, Wang S, Chen H, Mao Y, Chen T. Current and emerging therapies for primary central nervous system lymphoma. Biomark Res. 2021; 9:32.2. Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood. 2022; 140:971–9.3. Tu S, Zhou X, Guo Z, Huang R, Yue C, He Y, et al. CD19 and CD70 dual-target chimeric antigen receptor T-cell therapy for the treatment of relapsed and refractory primary central nervous system diffuse large B-cell lymphoma. Front Oncol. 2019; 9:1350.4. Karschnia P, Blobner J, Teske N, Schoberl F, Fitzinger E, Dreyling M, et al. CAR T-cells for CNS lymphoma: driving into new terrain? Cancers (Basel). 2021; 13:2503.5. Siddiqi T, Wang X, Blanchard MS, Wagner JR, Popplewell LL, Budde LE, et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 2021; 5:4059–63.6. Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q, et al. Radiation priming chimeric antigen receptor T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma with high tumor burden. J Immunother. 2020; 43:32–7.7. Frigault MJ, Dietrich J, Gallagher K, Roschewski M, Jordan JT, Forst D, et al. Safety and efficacy of tisagenlecleucel in primary CNS lymphoma: a phase 1/2 clinical trial. Blood. 2022; 139:2306–15.8. Jacobson CA, Falvey C, Bouvier R, Hogan S, Kendricken E, Jones J, et al. A pilot study of axicabtagene ciloleucel (axi-cel) for the treatment of relapsed/refractory primary and secondary central nervous system lymphoma (CNSL). Blood. 2022; 140(Suppl 1):1060–1.9. Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood. 2020; 136:1632–44.10. Li T, Zhao L, Zhang Y, Xiao Y, Wang D, Huang L, et al. CAR T-cell therapy is effective but not long-lasting in B-cell lymphoma of the brain. Front Oncol. 2020; 10:1306.11. Qu C, Zou R, Wang P, Zhu Q, Kang L, Ping N, et al. Decitabine-primed tandem CD19/CD22 CAR-T therapy in relapsed/refractory diffuse large B-cell lymphoma patients. Front Immunol. 2022; 13:969660.12. Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res. 2016; 22:2684–96.13. Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015; 112:E966–72.14. Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019; 6:e67–78.15. Cao Y, Lu W, Sun R, Jin X, Cheng L, He X, et al. Anti-CD19 chimeric antigen receptor T cells in combination with nivolumab are safe and effective against relapsed/refractory B-cell non-Hodgkin lymphoma. Front Oncol. 2019; 9:767.16. Zhang W, Huang C, Liu R, Zhang H, Li W, Yin S, et al. Case report: CD19-directed CAR-T cell therapy combined with BTK inhibitor and PD-1 antibody against secondary central nervous system lymphoma. Front Immunol. 2022; 13:983934.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD19-Specific CAR-T Cell Treatment of 115 Children and Young Adults with Acute B Lymphoblastic Leukemia: Long-term Follow-up
- Outcomes of Anti-CD19 CAR-T Treatment of Pediatric B-ALL with Bone Marrow and Extramedullary Relapse
- Primary Central Nervous System Lymphoma Mimicking Behcet's Disease
- Temporary Spontaneous Remission in Primary CNS Lymphoma
- Chimeric Antigen Receptor T-Cell Therapy for Diffuse Large B-Cell Lymphoma