Cancer Res Treat.
2023 Oct;55(4):1096-1103. 10.4143/crt.2022.1565.
A Multicenter, Prospective, Observational Study to Evaluate Ethanol-Induced Symptoms in Patients Receiving Docetaxel Chemotherapy
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 2Division of Oncology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Hematology and Oncology, Department of Internal Medicine, Chungnam National University Sejong Hospital, Chungnam National University College of Medicine, Sejong, Korea
- 4Division of Hematology/Oncology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Division of Hematology and Oncology, Department of Internal Medicine, Gyeongsang National University College of Medicine, Jinju, Korea
- 6Division of Oncology and Hematology, Department of Internal Medicine, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 7Division of Medical Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 8Division of Hematology and Oncology, Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 9Division of Hematology and Oncology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 10Division of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 11Division of Hematology and Oncology, Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan University College of Medicine, Yangsan, Korea
- 12Division of Hematology and Oncology, Department of Internal Medicine, Yeungnam University Hospital, Yeungnam University College of Medicine, Daegu, Korea
- 13Division of Hematology and Oncology, Department of Internal Medicine, Ulsan University Hospital, Ulsan University College of Medicine, Ulsan, Korea
- 14Center for Breast Cancer, Research Institute, National Cancer Center, Goyang, Korea
- 15Division of Hematology and Oncology, Department of Internal Medicine, Soonchunhyang University Hospital Bucheon, Soonchunhyang University College of Medicine, Bucheon, Korea
- 16Division of Hematology and Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 17Division of Hematology and Oncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 18Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 19Division of Hematology and Oncology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 20Division of Hematology and Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- KMID: 2547784
- DOI: http://doi.org/10.4143/crt.2022.1565
Abstract
- Purpose
Several previous studies and case reports have reported ethanol-induced symptoms in patients receiving anticancer drugs containing ethanol. Most docetaxel formulations contain ethanol as a solvent. However, there are insufficient data on ethanol-induced symptoms when docetaxel-containing ethanol is administered. The primary purpose of this study was to investigate the frequency and pattern of ethanol-induced symptoms during and after docetaxel administration. The secondary purpose was to explore the risk factors for ethanol-induced symptoms.
Materials and Methods
This was a prospective, multicenter, observational study. The participants filled out ethanol-induced symptom questionnaire on the day of chemotherapy and the following day.
Results
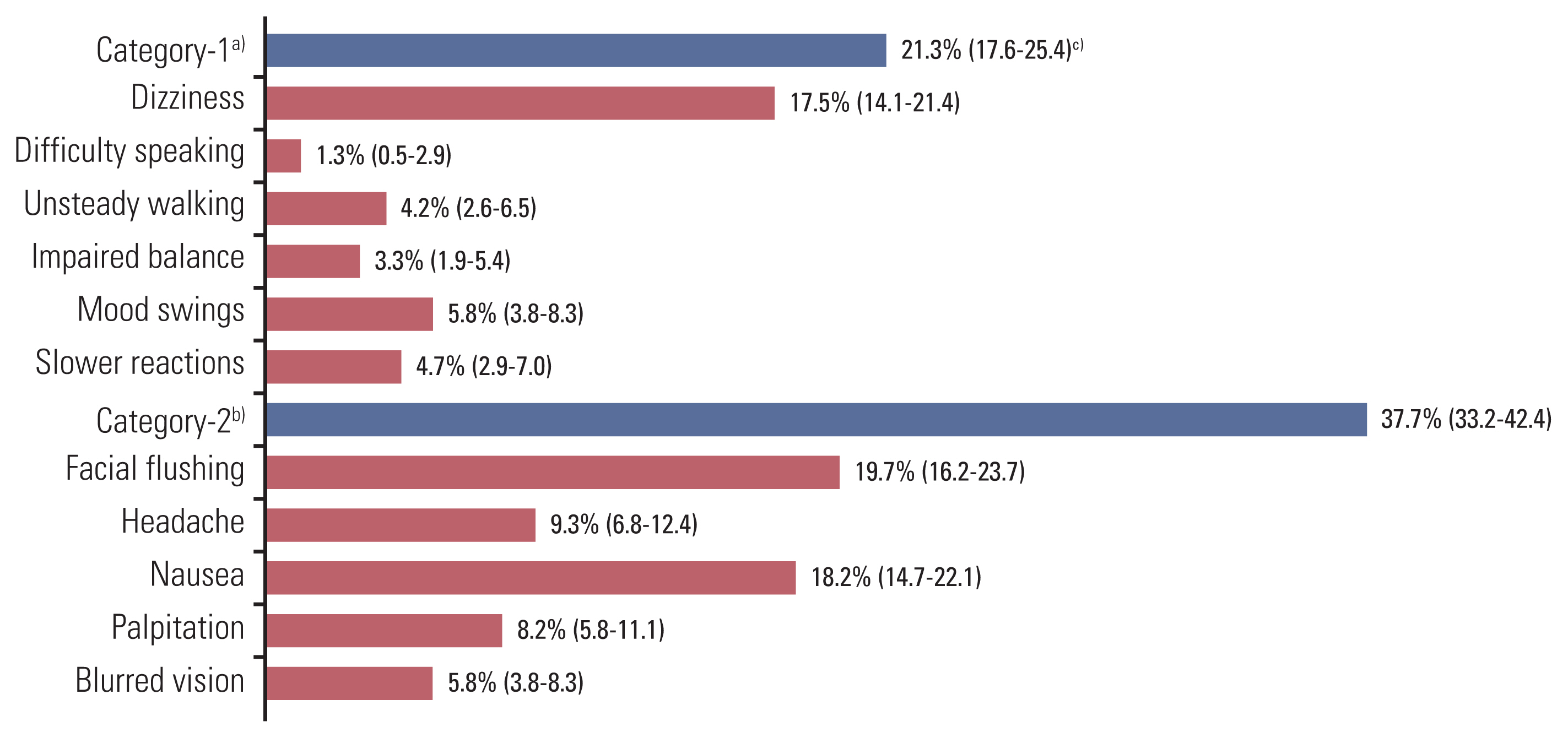
Data from 451 patients were analyzed. The overall occurrence rate of ethanol-induced symptoms was 44.3% (200/451 patients). The occurrence rate of facial flushing was highest at 19.7% (89/451 patients), followed by nausea in 18.2% (82/451 patients), and dizziness in 17.5% (79/451 patients). Although infrequent, unsteady walking and impaired balance occurred in 4.2% and 3.3% of patients, respectively. Female sex, presence of underlying disease, younger age, docetaxel dose, and docetaxel-containing ethanol amount were significantly associated with the occurrence of ethanol-induced symptoms.
Conclusion
The occurrence of ethanol-induced symptoms was not low in patients receiving docetaxel-containing ethanol. Physicians need to pay more attention to the occurrence of ethanol-induced symptoms and prescribe ethanol-free or low-ethanol-containing formulations to high-risk patients.
Keyword
Figure
Reference
-
References
1. Fries H, Hitzschke M, Lordick F. A different kind of relapse: ethanol as an additive in chemotherapy formulations. Oncol Res Treat. 2019; 42:350–3.2. Aomori T, Makino H, Sekizuka M, Hashita T, Araki T, Iizuka K, et al. Effect of ethanol in paclitaxel injections on the ethanol concentration in exhaled breath. Drugs R D. 2012; 12:165–70.3. Diez-Fernandez R, Vazquez-Sanchez R, Lopez-Esteban L, Enrech-Frances S, Sanchez-Pena AM, Diaz-Paniagua L, et al. Ethanol-induced symptoms in patients receiving gemcitabine diluted from a concentrate for solution for infusion containing ethanol. J Oncol Pharm Pract. 2018; 24:511–6.4. Mirza A, Mithal N. Alcohol intoxication with the new formulation of docetaxel. Clin Oncol (R Coll Radiol). 2011; 23:560–1.5. Webster LK, Crinis NA, Morton CG, Millward MJ. Plasma alcohol concentrations in patients following paclitaxel infusion. Cancer Chemother Pharmacol. 1996; 37:499–501.6. U.S. Food and Drug Administration. FDA Drug Safety Communications: FDA warns that cancer drug docetaxel may cause symptoms of alcohol intoxication after treatment. Silver Spring, MD: U.S. Food and Drug Administration;2016.7. Vonghia L, Leggio L, Ferrulli A, Bertini M, Gasbarrini G, Addolorato G, et al. Acute alcohol intoxication. Eur J Intern Med. 2008; 19:561–7.8. Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. Hoboken, NJ: John Wiley & Sons;2013.9. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998; 17:857–72.10. Searle J. Alcohol calculations and their uncertainty. Med Sci Law. 2015; 55:58–64.11. Hiver Q, Henry H, Vasseur M, Cuvelier E, Le Rhun E, Turpin A, et al. Ethanol exposure during the intravenous administration of chemotherapeutic drugs: an analysis of clinical practice and a literature review. JCO Oncol Pract. 2022; 18:e710–20.12. Yagi T, Fujiishi K, Hasegawa A, Otsuka T, Yoshinami T, Nishio M, et al. Aldehyde dehydrogenase 2 genotype in tolerability of alcohol contained in paclitaxel in Japanese breast cancer patients. Breast Cancer. 2019; 26:229–34.13. Iwahashi K, Suwaki H. Ethanol metabolism, toxicity and genetic polymorphism. Addict Biol. 1998; 3:249–59.14. Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012; 16:667–85.15. Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007; 17:239–57.16. Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001; 25:502–7.17. Kwo PY, Ramchandani VA, O’Connor S, Amann D, Carr LG, Sandrasegaran K, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998; 115:1552–7.18. Meier P, Seitz HK. Age, alcohol metabolism and liver disease. Curr Opin Clin Nutr Metab Care. 2008; 11:21–6.19. Thomasson HR. Gender differences in alcohol metabolism. Galanter M, Begleiter H, Deitrich R, Gallant D, Goodwin D, Gottheil E, editors. Recent development in alcoholism. 12. Boston, MA: Springer;2002. p. 163–79.20. Weathermon R, Crabb DW. Alcohol and medication interactions. Alcohol Res Health. 1999; 23:40–54.21. Lynch T, Price A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician. 2007; 76:391–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety Results of Docetaxel-(Taxotere(R))-Based Chemotherapy in Early Breast Cancer Patients of Asia-Pacific Region: Asia-Pacific Breast Initiative II
- Incidence of Febrile Neutropenia in Korean Female Breast Cancer Patients Receiving Preoperative or Postoperative Doxorubicin/Cyclophosphamide Followed by Docetaxel Chemotherapy
- Three Cases of Docetaxel-induced Acral Erythema
- Secondary Prophylaxis of Docetaxel Induced Diarrhea with Loperamide: Case Report
- Induction chemotherapy in patients with locally-advanced head and neck squamous cell carcinoma: docetaxel and cisplatin