Cancer Res Treat.
2023 Oct;55(4):1065-1076. 10.4143/crt.2023.846.
Recent Developments in the Therapeutic Landscape of Advanced or Metastatic Hormone Receptor–Positive Breast Cancer
- Affiliations
-
- 1Department of Medicine, University of California San Francisco, San Francisco, CA, USA
- 2Cancer Research Institute, Seoul National University, Seoul, Korea
- 3Translational Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2547781
- DOI: http://doi.org/10.4143/crt.2023.846
Abstract
- Hormone receptor–positive (HR+) disease is the most frequently diagnosed subtype of breast cancer. Among tumor subtypes, natural course of HR+ breast cancer is indolent with favorable prognosis compared to other subtypes such as human epidermal growth factor protein 2–positive disease and triple-negative disease. HR+ tumors are dependent on steroid hormone signaling and endocrine therapy is the main treatment option. Recently, the discovery of cyclin-dependent kinase 4/6 inhibitors and their synergistic effects with endocrine therapy has dramatically improved treatment outcome of advanced HR+ breast cancer. The demonstrated efficacy of additional nonhormonal agents, such as targeted therapy against mammalian target of rapamycin and phosphatidylinositol 3-kinase signaling, poly(ADP-ribose) polymerase inhibitors, antibody-drug conjugates, and immunotherapeutic agents have further expanded the available therapeutic options. This article reviews the latest advancements in the treatment of HR+ breast cancer, and in doing so discusses not only the development of currently available treatment regimens but also emerging therapies that invite future research opportunities in the field.
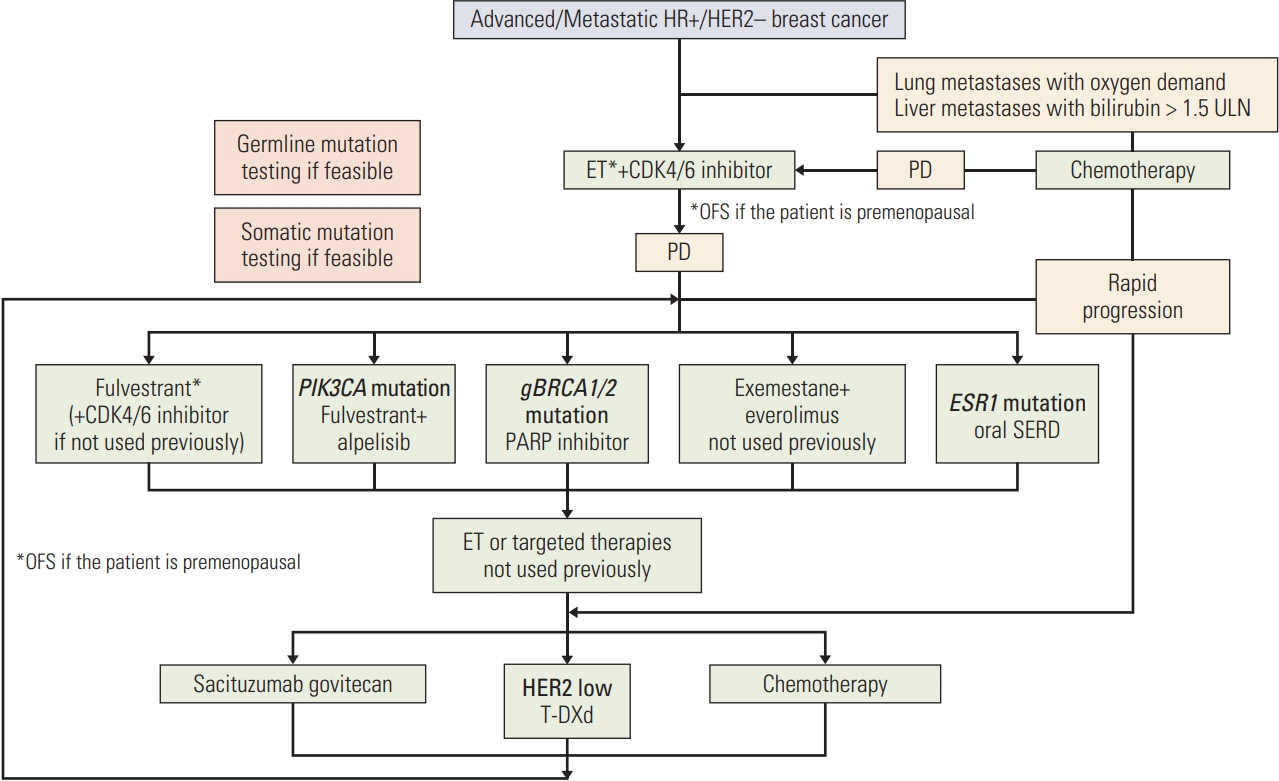
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–74.3. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22:1736–47.4. Zhang MH, Man HT, Zhao XD, Dong N, Ma SL. Estrogen receptor-positive breast cancer molecular signatures and therapeutic potentials (Review). Biomed Rep. 2014; 2:41–52.5. Huppert LA, Gumusay O, Idossa D, Rugo HS. Systemic therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative early stage and metastatic breast cancer. CA Cancer J Clin. 2023; 73:480–515.6. Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018; 186:1–24.7. Miller WR, Bartlett J, Brodie AM, Brueggemeier RW, di Salle E, Lonning PE, et al. Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter? Oncologist. 2008; 13:829–37.8. Gennari A, Andre F, Barrios CH, Cortes J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021; 32:1475–95.9. Scott SC, Lee SS, Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol. 2017; 44:385–94.10. Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptorpositive human breast cancer cell lines in vitro. Breast Cancer Res. 2009; 11:R77.11. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as firstline treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015; 16:25–35.12. Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375:1925–36.13. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016; 375:1738–48.14. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2- negative advanced breast cancer. Ann Oncol. 2018; 29:1541–7.15. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019; 381:307–16.16. Lu YS, Im SA, Colleoni M, Franke F, Bardia A, Cardoso F, et al. Updated Overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER2- advanced breast cancer in MONALEESA-7: a phase III randomized clinical trial. Clin Cancer Res. 2022; 28:851–9.17. Johnston S, Martin M, Di Leo A, Im SA, Awada A, Forrester T, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019; 5:5.18. Goetz MP, Toi M, Huober J, Sohn J, Tredan O, Park IH, et al. LBA15 MONARCH 3: interim overall survival (OS) results of abemaciclib plus a nonsteroidal aromatase inhibitor (NSAI) in patients (pts) with HR+, HER2- advanced breast cancer (ABC). Ann Oncol. 2022; 33:S1384.19. Slamon DJ, Neven P, Chia S, Jerusalem G, De Laurentiis M, Im S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol. 2021; 32:1015–24.20. Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015; 373:209–19.21. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017; 35:2875–84.22. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018; 19:904–15.23. Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016; 17:425–39.24. Finn RS, Rugo HS, Dieras VC, Harbeck N, Im SA, Gelmon KA et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J Clin Oncol. 2022; 40(17 Suppl):LBA1003.25. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022; 386:942–50.26. Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020; 6:116–24.27. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017; 35:3638–46.28. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018; 36:2465–72.29. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020; 18:452–78.30. Lu YS, Mahidin EI, Azim H, Eralp Y, Yap YS, Im SA, et al. Abstract GS1-10: primary results from the randomized phase II RIGHT choice trial of premenopausal patients with aggressive HR+/HER2− advanced breast cancer treated with ribociclib+endocrine therapy vs. physician’s choice combination chemotherapy. Cancer Res. 2023; 83(5 Suppl):GS1–10.31. McAndrew NP, Finn RS. Clinical review on the management of hormone receptor-positive metastatic breast cancer. JCO Oncol Pract. 2022; 18:319–27.32. Spring LM, Zangardi ML, Moy B, Bardia A. Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: practical considerations and recommendations. Oncologist. 2017; 22:1039–48.33. Thill M, Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018; 10:1758835918793326.34. Llombart-Cussac A, Perez-Garcia JM, Bellet M, Dalenc F, GilGil M, Ruiz-Borrego M, et al. Fulvestrant-palbociclib vs. letrozole-palbociclib as initial therapy for endocrine-sensitive, hormone receptor-positive, ERBB2-negative advanced breast cancer: a randomized clinical trial. JAMA Oncol. 2021; 7:1791–9.35. Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018; 379:1926–36.36. Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014; 23:489–502.37. Llombart-Cussac A, Sledge G, Toi M, Neven P, Sohn JH, Inoue K, et al. Abstract PD13-11: PD13-11 final overall survival analysis of Monarch 2 : a phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. Cancer Res. 2023; 83(5 Suppl):PD13–11.38. Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020; 382:514–24.39. Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017; 23:5218–24.40. Hamilton E, Cortes J, Ozyilkan O, Chen SC, Petrakova K, Manikhas A, et al. nextMONARCH: abemaciclib monotherapy or combined with tamoxifen for metastatic breast cancer. Clin Breast Cancer. 2021; 21:181–90.41. Elliott MJ, Cescon DW. Development of novel agents for the treatment of early estrogen receptor positive breast cancer. Breast. 2022; 62(Suppl 1):S34–42.42. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012; 490:61–70.43. Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptorpositive advanced breast cancer. N Engl J Med. 2019; 380:1929–40.44. Rugo HS, Lerebours F, Ciruelos E, Drullinsky P, Ruiz-Borrego M, Neven P, et al. Alpelisib plus fulvestrant in PIK3CAmutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021; 22:489–98.45. Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020; 21:345–57.46. Howell SJ, Casbard A, Carucci M, Ingarfield K, Butler R, Morgan S, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022; 23:851–64.47. Turner NC, Oliveira M, Howell SJ, Dalenc F, Cortes J, Gomez Moreno HL, et al. Capivasertib in hormone receptor-positive advanced breast cancer. N Engl J Med. 2023; 388:2058–70.48. Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormonereceptor-positive advanced breast cancer. N Engl J Med. 2012; 366:520–9.49. Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, et al. Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013; 30:870–84.50. Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormonereceptor-positive, human epidermal growth factor receptor2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014; 25:2357–62.51. Chandarlapaty S, Chen D, He W, Sung P, Samoila A, You D, et al. Prevalence of ESR1 mutations in cell-free DNA and outcomes in metastatic breast cancer: a secondary analysis of the BOLERO-2 clinical trial. JAMA Oncol. 2016; 2:1310–5.52. Bidard FC, Kaklamani VG, Neven P, Streich G, Montero AJ, Forget F, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022; 40:3246–56.53. Corti C, De Angelis C, Bianchini G, Malorni L, Giuliano M, Hamilton E, et al. Novel endocrine therapies: what is next in estrogen receptor positive, HER2 negative breast cancer? Cancer Treat Rev. 2023; 117:102569.54. Oliveira M, Pominchuck D, Nowecki Z, Hamilton E, Kulyaba Y, Andabekov T, et al. Abstract GS3-02: GS3-02 camizestrant, a next generation oral SERD vs. fulvestrant in post-menopausal women with advanced ER-positive HER2-negative breast cancer: results of the randomized, multi-dose phase 2 SERENA-2 trial. Cancer Res. 2023; 83(5 Suppl):GS3–02.55. Im SA, Hamilton EP, Llombart Cussac A, Baird RD, Ettl J, Goetz MP, et al. SERENA-4: a phase 3 comparison of AZD9833 (camizestrant) plus palbociclib, versus anastrozole plus palbociclib, for patients with ER-positive, HER2-negative advanced breast cancer who have not previously received systemic treatment for advanced disease. J Clin Oncol. 2021; 39(15 Suppl):TPS1101.56. Bidard FC, Kalinsky K, Cristofanilli M, Bianchini G, Chia SK, Janni W, et al. Abstract OT2-11-05: SERENA-6: a phase III study to assess the efficacy and safety of AZD9833 (camizestrant) compared with aromatase inhibitors when given in combination with palbociclib or abemaciclib in patients with HR+/HER2- metastatic breast cancer with detectable ESR1m who have not experienced disease progression on first-line therapy. Cancer Res. 2022; 82(4 Suppl):OT2–11.57. Jimenez MM, Lim E, Gregor MC, Bardia A, Wu J, Zhang Q, et al. 211MO Giredestrant (GDC-9545) vs. physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2– locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol. 2022; 33(Suppl 7):S633–4.58. Patel R, Klein P, Tiersten A, Sparano JA. An emerging generation of endocrine therapies in breast cancer: a clinical perspective. NPJ Breast Cancer. 2023; 9:20.59. Ferraro E, Walsh EM, Tao JJ, Chandarlapaty S, Jhaveri K. Accelerating drug development in breast cancer: new frontiers for ER inhibition. Cancer Treat Rev. 2022; 109:102432.60. Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015; 7:a016600.61. Turk AA, Wisinski KB. PARP inhibitors in breast cancer: bringing synthetic lethality to the bedside. Cancer. 2018; 124:2498–506.62. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012; 30:2654–63.63. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.64. Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019; 30:558–66.65. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.66. Zhang H, Katerji H, Turner BM, Hicks DG. HER2-low breast cancers. Am J Clin Pathol. 2022; 157:328–36.67. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016; 107:1039–46.68. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020; 38:1887–96.69. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022; 387:9–20.70. Liu X, Deng J, Yuan Y, Chen W, Sun W, Wang Y, et al. Advances in Trop2-targeted therapy: novel agents and opportunities beyond breast cancer. Pharmacol Ther. 2022; 239:108296.71. Kalinsky K, Diamond JR, Vahdat LT, Tolaney SM, Juric D, O’Shaughnessy J, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase I/II, single-arm, basket trial. Ann Oncol. 2020; 31:1709–18.72. Rugo HS, Bardia A, Marme F, Cortes J, Schmid P, Loirat D, et al. Primary results from TROPiCS-02: a randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor– positive/HER2-negative (HR+/HER2-) advanced breast cancer. J Clin Oncol. 2022; 40(17 Suppl):LBA1001.73. Rugo HS, Bardia A, Marme F, Cortes J, Schmid P, Loirat D, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 Aug 23 [Epub]. https://doi.org/10.1016/S0140-6736(23)01245-X.74. Rugo HS, Delord JP, Im SA, Ott PA, Piha-Paul SA, Bedard PL, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018; 24:2804–11.75. Chaudhuri S, Thomas S, Munster P. Immunotherapy in breast cancer: a clinician’s perspective. J Natl Cancer Cent. 2021; 1:47–57.76. Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, VazLuis I, et al. Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2020; 6:1598–605.77. Shah AN, Flaum L, Helenowski I, Santa-Maria CA, Jain S, Rademaker A, et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J Immunother Cancer. 2020; 8:e000173.78. Perez-Garcia JM, Llombart-Cussac A, Cortes MG, Curigliano G, Lopez-Miranda E, Alonso JL, et al. Pembrolizumab plus eribulin in hormone-receptor-positive, HER2-negative, locally recurrent or metastatic breast cancer (KELLY): an openlabel, multicentre, single-arm, phase Ⅱ trial. Eur J Cancer. 2021; 148:382–94.79. Kim SH, Suh KJ, Im SA, Lee KH, Kim MH, Sohn J, et al. A phase IB/II study of nivolumab in combination with eribulin in HER2-negative metastatic breast cancer (KCSG BR18-16). J Clin Oncol. 2022; 40(16 Suppl):1098.80. Rugo HS, Sohn J, Jerez Gilarranz Y, Gonzalez-Cortijo L, Sonnenblick A, Sabanathan D, et al. KEYNOTE-B49: a phase 3, randomized, double-blind, placebo-controlled study of pembrolizumab plus chemotherapy in patients with HR+/HER2- locally recurrent inoperable or metastatic breast cancer. J Clin Oncol. 2022; 40(16 Suppl):TPS1118.81. Yuan Y, Lee JS, Yost SE, Frankel PH, Ruel C, Egelston CA, et al. Phase I/II trial of palbociclib, pembrolizumab and letrozole in patients with hormone receptor-positive metastatic breast cancer. Eur J Cancer. 2021; 154:11–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hormone Treatment for Breast Cancer
- Hormone Therapy for Metastatic Breast Cancer
- Diagnosis and Treatment of HER2-Positive Breast Cancer
- Personalized therapy for advanced breast cancer using molecular signatures
- The Therapeutic Effect of Cyclin-Dependent Kinase 4/6 Inhibitor on Relapsed Ectopic Male Breast Cancer