J Korean Neurosurg Soc.
2023 Nov;66(6):618-631. 10.3340/jkns.2023.0061.
The Unique Relationship between Neuro-Critical Care and Critical Illness-Related Corticosteroid Insufficiency : Implications for Neurosurgeons in Neuro-Critical Care
- Affiliations
-
- 1Department of Neurological Surgery and Critical Care Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 2Department of Neurological Surgery and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Neurological Surgery and Critical Care Medicine, Chungnam National University Hospital, Daejeon, Korea
- 4Department of Neurological Surgery and Critical Care Medicine, Seoul National University Hospital, Seoul, Korea
- 5Department of Neurological Surgery and Critical Care Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Neurological Surgery and Critical Care Medicine, Kangbuk Samsung Hospital, Seoul, Korea
- 7Department of Neurological Surgery and Critical Care Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 8Department of Neurological Surgery and Critical Care Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 9Department of Neurological Surgery and Critical Care Medicine, Yeungnam University Medical Center, Daegu, Korea
- 10Department of Neurological Surgery and Critical Care Medicine, Gachon University Gil Hospital Regional Trauma Center, Incheon, Korea
- 11Department of Neurological Surgery and Critical Care Medicine, Gangnam Severance Hospital, Seoul, Korea
- 12Department of Neurological Surgery and Critical Care Medicine, Incheon St. Mary’s Hospital, The Catholic University of Korea, Incheon, Korea
- KMID: 2547456
- DOI: http://doi.org/10.3340/jkns.2023.0061
Abstract
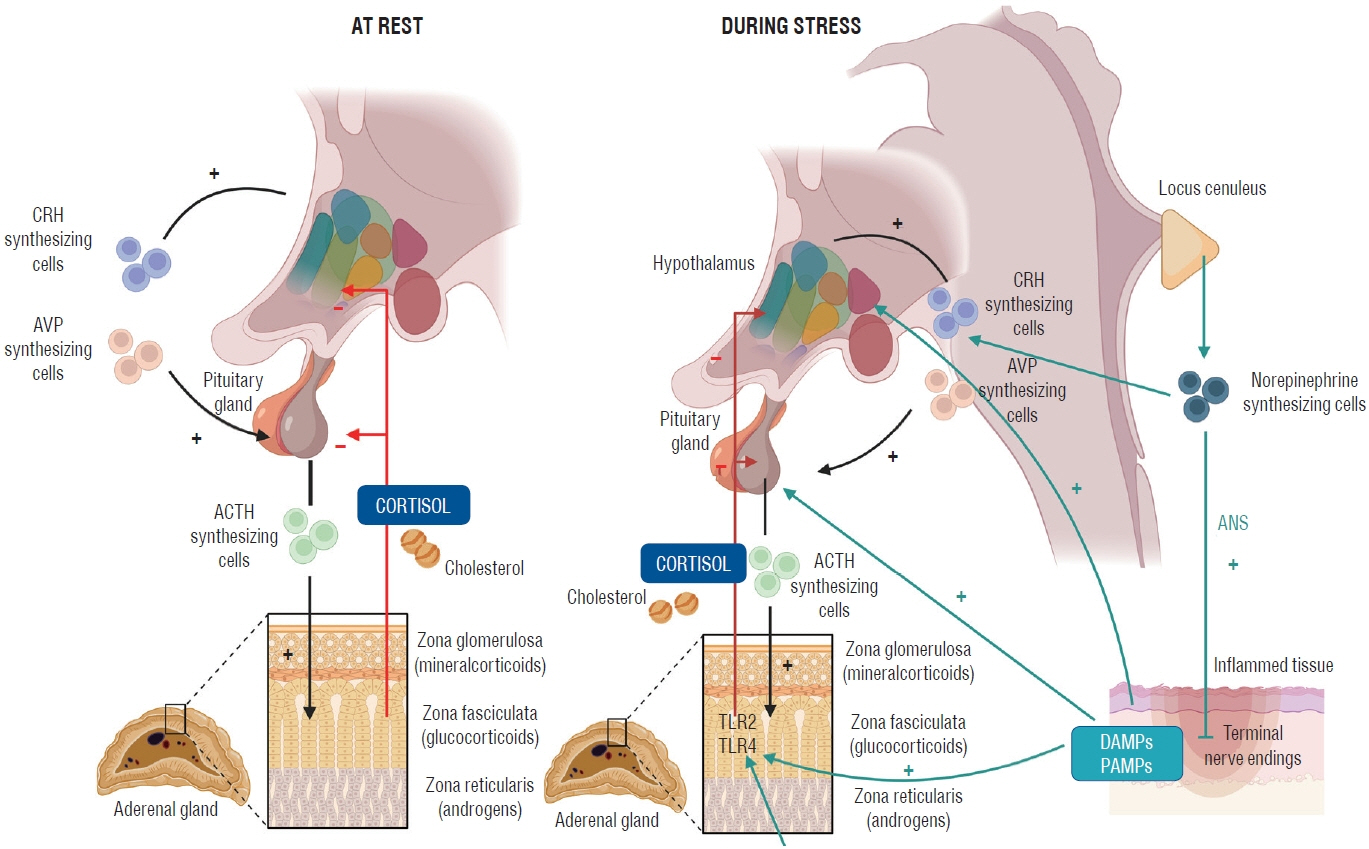
- The brain houses vital hormonal regulatory structures such as the hypothalamus and pituitary gland, which may confer unique susceptibilities to critical illness-related corticosteroid insufficiency (CIRCI) in patients with neurological disorders. In addition, the frequent use of steroids for therapeutic purposes in various neurological conditions may lead to the development of steroid insufficiency. This abstract aims to highlight the significance of understanding these relationships in the context of patient care and management for physicians. Neurological disorders may predispose patients to CIRCI due to the role of the brain in hormonal regulation. Early recognition of CIRCI in the context of neurological diseases is essential to ensure prompt and appropriate intervention. Moreover, the frequent use of steroids for treating neurological conditions can contribute to the development of steroid insufficiency, further complicating the clinical picture. Physicians must be aware of these unique interactions and be prepared to evaluate and manage patients with CIRCI and steroid insufficiency in the context of neurological disorders. This includes timely diagnosis, appropriate steroid administration, and careful monitoring for potential adverse effects. A comprehensive understanding of the interplay between neurological disease, CIRCI, and steroid insufficiency is critical for optimizing patient care and outcomes in this complex patient population.
Keyword
Figure
Reference
-
References
1. Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a multispecialty task force of the society of critical care medicine (SCCM) and the european society of intensive care medicine (ESICM). Intensive Care Med. 43:1781–1792. 2017.
Article2. Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illnessrelated corticosteroid insufficiency (CIRCI) in critically ill patients (part I): society of critical care medicine (SCCM) and european society of intensive care medicine (ESICM) 2017. Intensive Care Med. 43:1751–1763. 2017.
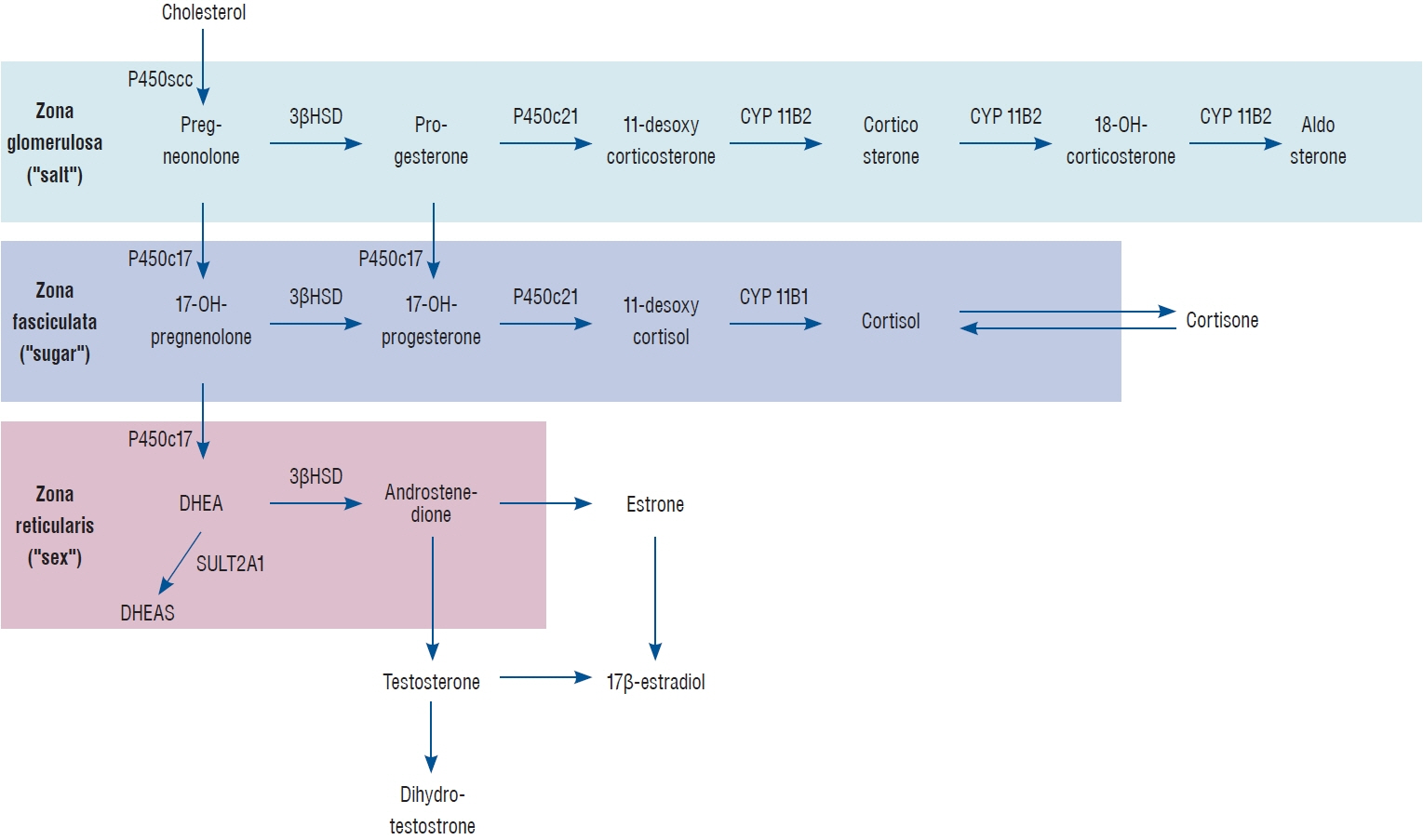
Article3. Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 34:293–313, viii. 2005.
Article4. Bendel S, Koivisto T, Ruokonen E, Rinne J, Romppanen J, Vauhkonen I, et al. Pituitary-adrenal function in patients with acute subarachnoid haemorrhage: a prospective cohort study. Crit Care. 12:R126. 2008.
Article5. Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 100:2171–2180. 2015.6. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 80:6–15. 2017.
Article7. Cohan P, Wang C, McArthur DL, Cook SW, Dusick JR, Armin B, et al. Acute secondary adrenal insufficiency after traumatic brain injury: a prospective study. Crit Care Med. 33:2358–2366. 2005.
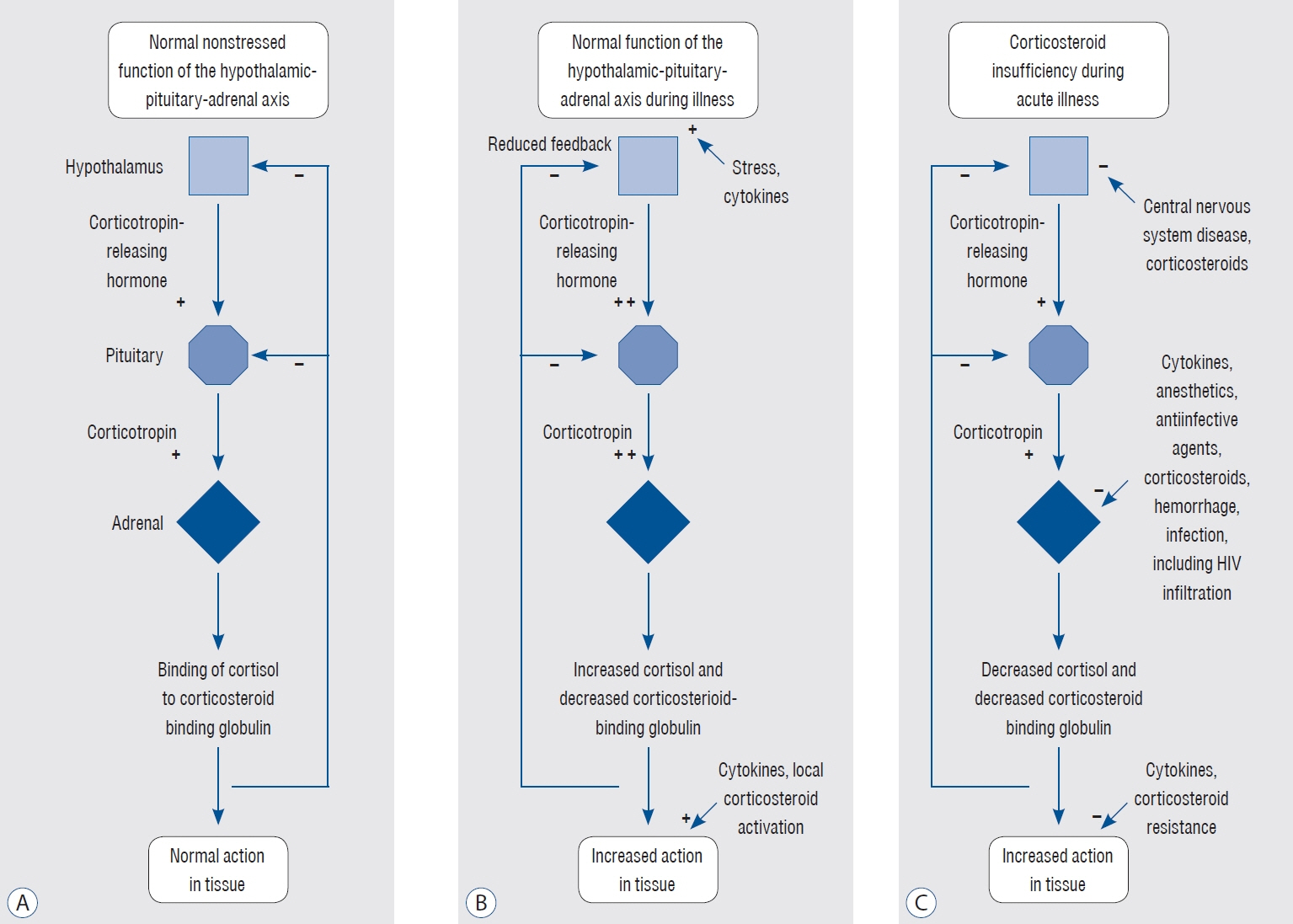
Article8. Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 348:727–734. 2003.
Article9. Dimopoulou I, Tsagarakis S, Kouyialis AT, Roussou P, Assithianakis G, Christoforaki M, et al. Hypothalamic-pituitary-adrenal axis dysfunction in critically ill patients with traumatic brain injury: incidence, pathophysiology, and relationship to vasopressor dependence and peripheral interleukin-6 levels. Crit Care Med. 32:404–408. 2004.
Article10. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47:1181–1247. 2021.11. Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 53:e282–e361. 2022.
Article12. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 46:133–141. 2016.
Article13. Karamouzis I, Pagano L, Prodam F, Mele C, Zavattaro M, Busti A, et al. Clinical and diagnostic approach to patients with hypopituitarism due to traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and ischemic stroke (IS). Endocrine. 52:441–450. 2016.
Article14. Laugesen K, Broersen LHA, Hansen SB, Dekkers OM, Sørensen HT, Jorgensen JOL. Management of endocrine disease: glucocorticoid-induced adrenal insufficiency: replace while we wait for evidence? Eur J Endocrinol. 184:R111–R122. 2021.
Article15. Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 9:30. 2013.
Article16. Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 36:1937–1949. 2008.
Article17. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 50:e344–e418. 2019.
Article18. Powner DJ, Boccalandro C. Adrenal insufficiency following traumatic brain injury in adults. Curr Opin Crit Care. 14:163–166. 2008.
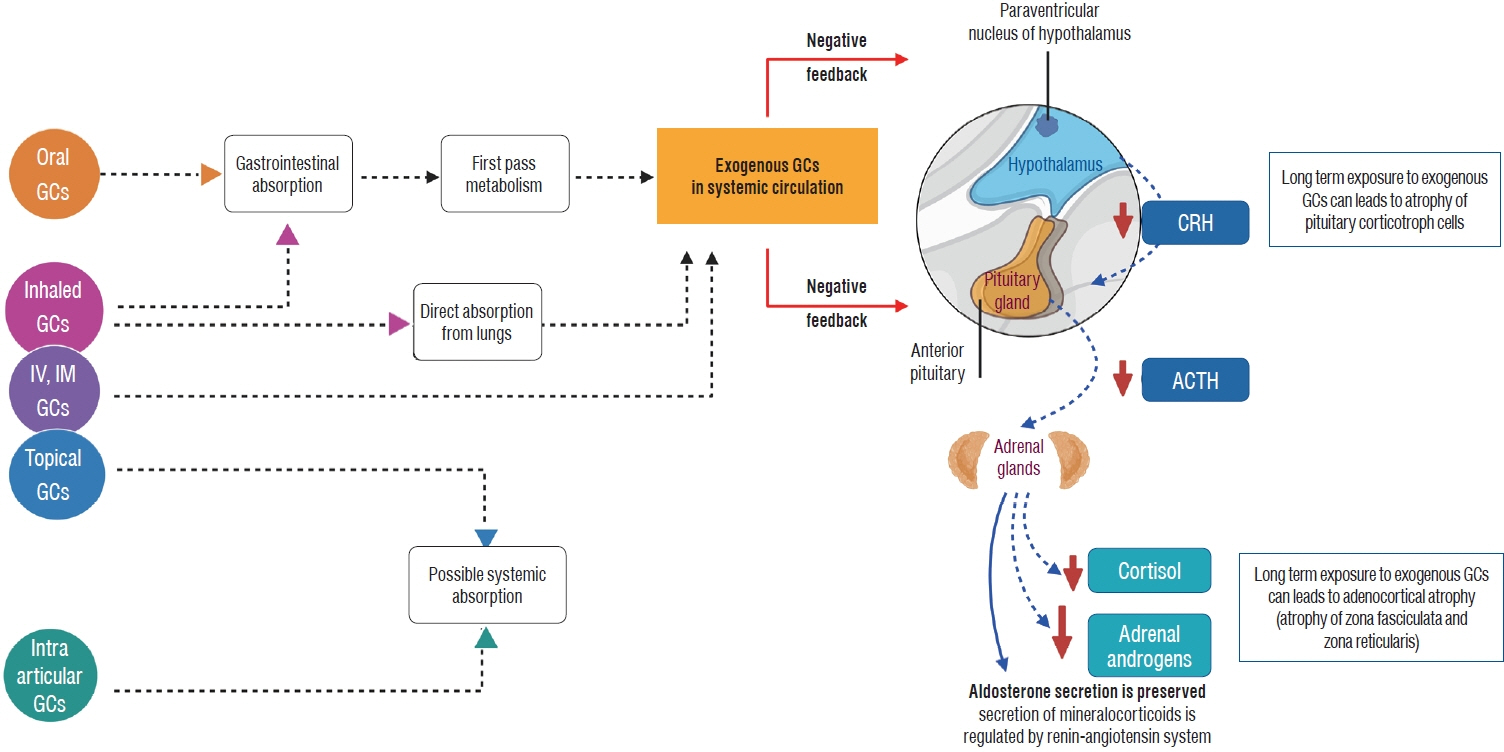
Article19. Prete A, Bancos I. Glucocorticoid induced adrenal insufficiency. BMJ. 374:n1380. 2021.
Article20. Schneider HJ, Kreitschmann-Andermahr I, Ghigo E, Stalla GK, Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 298:1429–1438. 2007.
Article21. Tanriverdi F, De Bellis A, Bizzarro A, Sinisi AA, Bellastella G, Pane E, et al. Antipituitary antibodies after traumatic brain injury: is head traumainduced pituitary dysfunction associated with autoimmunity? Eur J Endocrinol. 159:7–13. 2008.
Article22. Tanriverdi F, De Bellis A, Ulutabanca H, Bizzarro A, Sinisi AA, Bellastella G, et al. A five year prospective investigation of anterior pituitary function after traumatic brain injury: is hypopituitarism long-term after head trauma associated with autoimmunity? J Neurotrauma. 30:1426–1433. 2013.
Article23. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 8:267–276. 2020.
Article24. Weant KA, Sasaki-Adams D, Dziedzic K, Ewend M. Acute relative adrenal insufficiency after aneurysmal subarachnoid hemorrhage. Neurosurgery. 63:645–649. discussion 649-650. 2008.
Article25. Wijdicks EF, Findlay JY, Freeman WD, Ayan Sen M : Mayo clinic critical and neurocritical care board review. Oxford : Oxford University Press, 2019.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Critical illness-related corticosteroid insufficiency: latest pathophysiology and management guidelines
- Corticosteroid Treatment in Critically Ill Patients
- Neurocritical Care Nutrition: Unique Considerations and Strategies for Optimizing Energy Supply and Metabolic Support in Critically Ill Patients
- Critical Care Research Using “Big Dataâ€: A Reality in the Near Future
- Influence of sarcopenia focused on critically ill patients