Korean J Schizophr Res.
2023 Oct;26(2):52-60. 10.16946/kjsr.2023.26.2.52.
An International Adult Guideline for Making Clozapine Titration Safer by Using Ancestry-Based Personalized Dosing Titrations, C-Reactive Protein and Serum Clozapine Levels: Korean Translation and Its Clinical Application Cases
- Affiliations
-
- 1Department of Psychiatry, Seoul National University Hospital, Seoul, Korea
- 2Department of Psychiatry, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Psychiatry, Eulji University Hospital, Seoul, Korea
- KMID: 2547422
- DOI: http://doi.org/10.16946/kjsr.2023.26.2.52
Abstract
Objectives
During the initial clozapine titration, it is crucial to monitor for inflammatory reactions to ensure safe and effective administration. Clozapine metabolism varies by ancestry, particularly among Asians, warranting lower dosage. Recently, Dr. De Leon introduced guidelines based on ancestral differences. We aimed to provide a Korean translation, focusing on illustrating the necessity through clinical cases.
Methods
The Korean translation of the guidelines, approved by Dr. De Leon, involved two psychiatrists who reviewed and revised each other’s work. An additional board-certified psychiatrist conducted an independent review. We examined two clinical cases from our institution’s database, where clozapine titration faced challenges due to fever and pneumonia, assessing guideline applicability.
Results
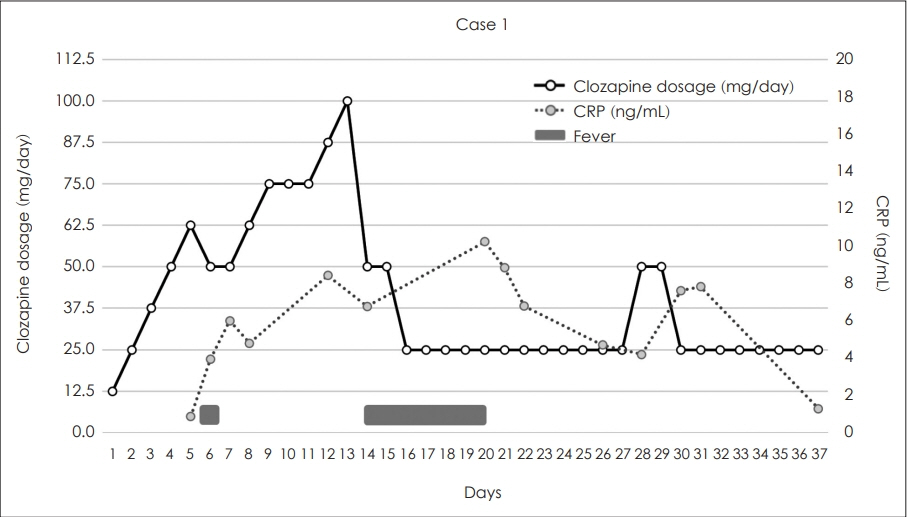
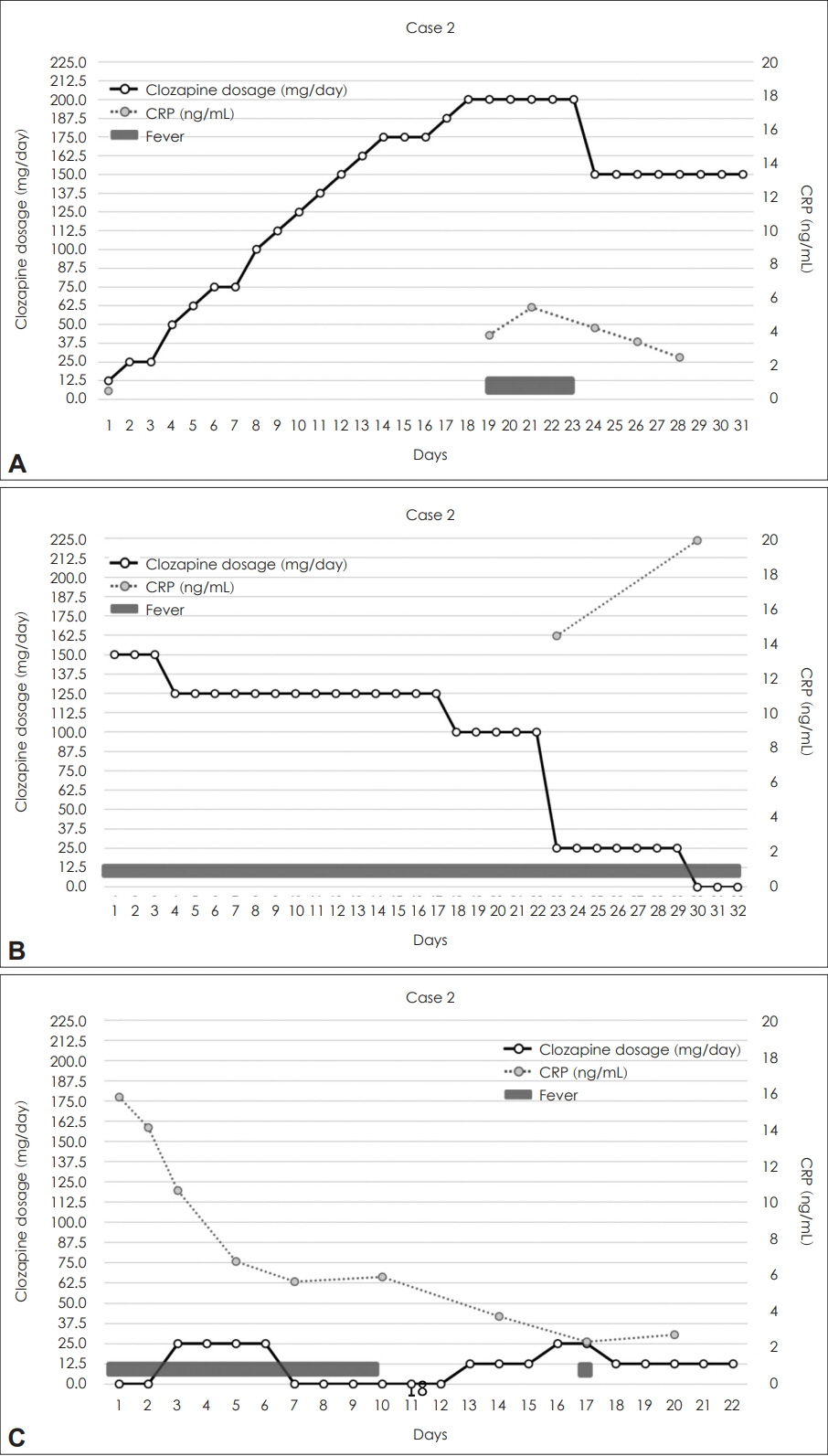
The guidelines recommend a target clozapine dose of 175-300 mg/day for Asians with average metabolism and a slower titration rate compared to other ancestries. In both cases, CRP elevation was detected either before or simultaneously with the onset of fever, with a concentration-to-dose ratio ranging from 3.06 to 6.97.
Conclusion
The initial clozapine titration process should consider metabolic differences by ancestry and monitor for inflammation. Further research is needed to optimize the titration process for Koreans, considering metabolic rates, usage patterns, and side effects.
Keyword
Figure
Reference
-
1. Howes OD, McCutcheon R, Agid O. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on diagnosis and terminology. Am J Psychiatry. 2017; 174:216–229.
Article2. Siskind D, McCartney L, Goldschlager R, Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016; 209:385–392.
Article3. Meltzer HY, Alphs L, Green AI. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003; 60:82–91.
Article4. Citrome L, Volavka J. Specific anti-hostility effects of atypical antipsychotics in persons with schizophrenia: from clozapine to cariprazine. Harv Rev Psychiatry. 2021; 29:20–34.
Article5. Bachmann CJ, Aagaard L, Bernardo M. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017; 136:37–51.
Article6. Kane J, Honigfeld G, Singer J. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988; 45:789–796.7. de Leon J, Sanz EJ, Norén GN. Pneumonia may be more frequent and have more fatal outcomes with clozapine than with other second-generation antipsychotics. World Psychiatry. 2020; 19:120–121.
Article8. Rohde C, Siskind D, de Leon J. Antipsychotic medication exposure, clozapine, and pneumonia: results from a self-controlled study. Acta Psychiatr Scand. 2020; 142:78–86.
Article9. Villasante-Tezanos AG, Rohde C, Nielsen J. Pneumonia risk: approximately one-third is due to clozapine and two-thirds is due to treatment-resistant schizophrenia. Acta Psychiat Scand. 2020; 142:66–67.
Article10. Schoretsanitis G, Ruan CJ, Rohde C. An update on the complex relationship between clozapine and pneumonia. Expert Rev Clin Pharmacol. 2021; 24:1–5.
Article11. Vohra J. Sudden cardiac death in schizophrenia: a review. Heart Lung Circ. 2020; 29:1427–1432.
Article12. Papola D, Ostuzzi G, Gastaldon C. Antipsychotic use and risk of life- threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand. 2019; 140:227–243.
Article13. Myles N, Myles H, Xia S. A meta-analysis of controlled studies comparing the association between clozapine and other antipsychotic medications and the development of neutropenia. Aust N Z J Psychiatry. 2019; 53:403–412.
Article14. Wiciński M, Węclewicz MM. Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr Opin Hematol. 2018; 25:22–28.
Article15. Winckel K, Siskind D, Hollingworth S. Clozapine-induced myocarditis: separating the wheat from the chaff. Aust N Z J Psychiatry. 2015; 49:188.
Article16. Ronaldson KJ, Fitzgerald PB, Taylor AJ. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust NZ J Psychiatry. 2011; 45:458–465.
Article17. Ronaldson KJ, Fitzgerald PB, Taylor AJ. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012; 141:173–178.
Article18. Shirazi A, Stubbs B, Gomez L. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016; 17:863.
Article19. West S, Rowbotham D, Xiong G. Clozapine induced gastrointestinal hypomotility: a potentially life threatening adverse event. A review of the literature. Gen Hosp Psychiatry. 2017; 46:32–37.
Article20. Cohen D. Clozapine and gastrointestinal hypomotility. CNS Drugs. 2017; 31:1083–1091.
Article21. de Leon J, Schoretsanitis G, Kane JM. Using therapeutic drug monitoring to personalize clozapine dosing in Asians. Asia Pac Psychiatry. 2020; 12:e12384.
Article22. Spina E, de Leon J. Clinically relevant interactions between newer antidepressants and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2014; 10:721–746.
Article23. Pacia SV, Devinsky O. Clozapine-related seizures: experience with 5,629 patients. Neurology. 1994; 44:2247–2249.
Article24. Clancy MJ, Clarke MC, Connor DJ. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014; 14:75.
Article25. Asenjo Lobos C, Komossa K, Rummel-Kluge C. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010; CD006633.
Article26. Trifirò G, Spina E. Age-related changes in pharmacodynamics: focus on drugs acting on central nervous and cardiovascular systems. Curr Drug Metab. 2011; 12:611–620.
Article27. Nielsen J, Correll CU, Manu P. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013; 74:603–613.28. Sabaawi M, Singh NN, de Leon J. Guidelines for the use of clozapine in individuals with developmental disabilities. Res Dev Disabil. 2006; 27:309–336.
Article29. de Leon J, Ruan CJ, Schoretsanitis G. A rational use of clozapine based on adverse drug reactions, pharmacokinetics, and clinical pharmacopsychology. Psychother Psychosom. 2020; 89:200–204.
Article30. Bertilsson L, Carrillo JA, Dahl ML. Clozapine disposition covaries with CYP1A2 activity determined by a caffeine test. Br J Clin Pharmacol. 1994; 38:471–473.
Article31. Perry PJ, Bever KA, Arndt S. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry. 1998; 44:733–738.
Article32. de Leon J. Future studies on the interaction between clozapine and valproic acid should aspire to include longitudinal designs and free valproate concentrations, and should consider that inducer and/or inhibitory effects may vary with time, the individual, and the autoinduction of valproic acid. Ther Drug Monit. 2020; 42:159–161.
Article33. Clark SR, Warren NS, Kim G. Elevated clozapine levels associated with infection: a systematic review. Schizophr Res. 2018; 192:50–56.
Article34. Diaz FJ, Josiassen RC, de Leon J. The effect of body weight changes on total plasma clozapine concentrations determined by applying a statistical model to the data from a double-blind trial. J Clin Psychopharmacol. 2018; 38:442–446.
Article35. Ruan CJ, Zang YN, Cheng YH. Around 3% of 1,300 levels were elevated during infections in a retrospective review of 131 Beijing hospital in-patients with more than 24,000 days of clozapine treatment. Psychother Psychosom. 2020; 89:255–257.
Article36. Hiemke C, Bergemann N, Clement HW. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018; 51:9–62.37. de Leon J, Schoretsanitis G, Smith RL, Molden E, Solismaa A, Seppälä N, et al. An international adult guideline for making clozapine titration safer by using six ancestry-based personalized dosing titrations, CRP, and clozapine levels. Pharmacopsychiatry. 2022; 55:73–86.38. de Leon J, Rhee DW, Kondracke A. Rapid titration and decreased clozapine clearance may help explain five cases of clozapine-induced myocarditis in a New York Hospital. Psychosomatics. 2020; 61:102–103.
Article39. Danilewitz M, Rafizadeh R, Bousman CA. Successful clozapine rechallenge after suspected clozapine-associated myocarditis: a case report. J Clin Psychopharmacol. 2021; 41:218–220.
Article40. Verdoux H, Quiles C, de Leon J. Clinical determinants of fever in clozapine users and implications for treatment management: a narrative review. Schizophr Res. 2019; 211:1–9.
Article41. Schoretsanitis G, Kane JM, Correll CU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020; 81:19. cs13169.42. Williams RL. FDA position on product selection for “narrow therapeutic index” drugs. Am J Health Syst Pharm. 1997; 54:1630–1632.
Article43. Spina E, Hiemke C, de Leon J. Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2016; 12:407–422.
Article44. Cohen D, Bogers JP, van Dijk D. Beyond white blood cell monitoring: screening in the initial phase of clozapine therapy. J Clin Psychiatry. 2012; 73:1307–1312.45. de Leon J, Sanz EJ, De Las Cuevas C. Data from the World Health Organization’s pharmacovigilance database supports the prominent role of pneumonia in mortality associated with clozapine adverse drug reactions. Schizophr Bull. 2020; 46:1–3.
Article46. Rohde C, Polcwiartek C, Kragholm K. Adverse cardiac events in out-patients initiating clozapine treatment: a nationwide register-based study. Acta Psychiatr Scand. 2018; 137:47–53.
Article47. Ifteni P, Correll CU, Nielsen J. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014; 166:168–172.
Article48. Sherwood M, Thornton AE, Honer WG. A quantitative review of the profile and time course of symptom change in schizophrenia treated with clozapine. Journal of Psychopharmacology. 2012; 26:1175–1184.
Article49. Kim SH. Current status of clozapine for treatment-resistant schizophrenia. Korean Journal of Schizophrenia Research. 2021; 24:1–7.
Article50. Kim CE, Lee YH, Lee KH, Kang MH. Clozapine dosage and blood concentrations of Korean adult schizophrenic patients. Journal of Korean Neuropsychiatric Association. 2001; 109–117.51. Lee ST, Ryu S, Nam HJ, Lee SY, Hong KS. Determination of pharmacokinetic properties of clozapine and norclozapine in Korean schizophrenia patients. International clinical psychopharmacology. 2009; 24:139–144.
Article52. Pollmächer T, Haack M, Schuld A, Kraus T, Hinze-Selch D. Effects of antipsychotic drugs on cytokine networks. Journal of psychiatric research. 2000; 34:369–382.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reflections on the Lack of Consideration of Ethnic Ancestry to Stratify Clozapine Dosing
- A Case of Acute Pancreatitis and Severe Hypertriglyceridemia Associated with Clozapine
- Effect of Clozapine on Plasma Prolactin Levels in Schizophrenic Patients
- Fever Associated with Clozapine Administration: Incidence, Clinical Characteristics, and Related Factors
- The Efficacy and Safety of Concurrent Administration of Clozapine and Electroconvulsive Therapy: A Case Report