Neurointervention.
2023 Nov;18(3):149-158. 10.5469/neuroint.2023.00339.
Proposed Protocols for Artificial Intelligence Imaging Database in Acute Stroke Imaging
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2547276
- DOI: http://doi.org/10.5469/neuroint.2023.00339
Abstract
- Purpose
To propose standardized and feasible imaging protocols for constructing artificial intelligence (AI) database in acute stroke by assessing the current practice at tertiary hospitals in South Korea and reviewing evolving AI models.
Materials and Methods
A nationwide survey on acute stroke imaging protocols was conducted using an electronic questionnaire sent to 43 registered tertiary hospitals between April and May 2021. Imaging protocols for endovascular thrombectomy (EVT) in the early and late time windows and during follow-up were assessed. Clinical applications of AI techniques in stroke imaging and required sequences for developing AI models were reviewed. Standardized and feasible imaging protocols for data curation in acute stroke were proposed.
Results
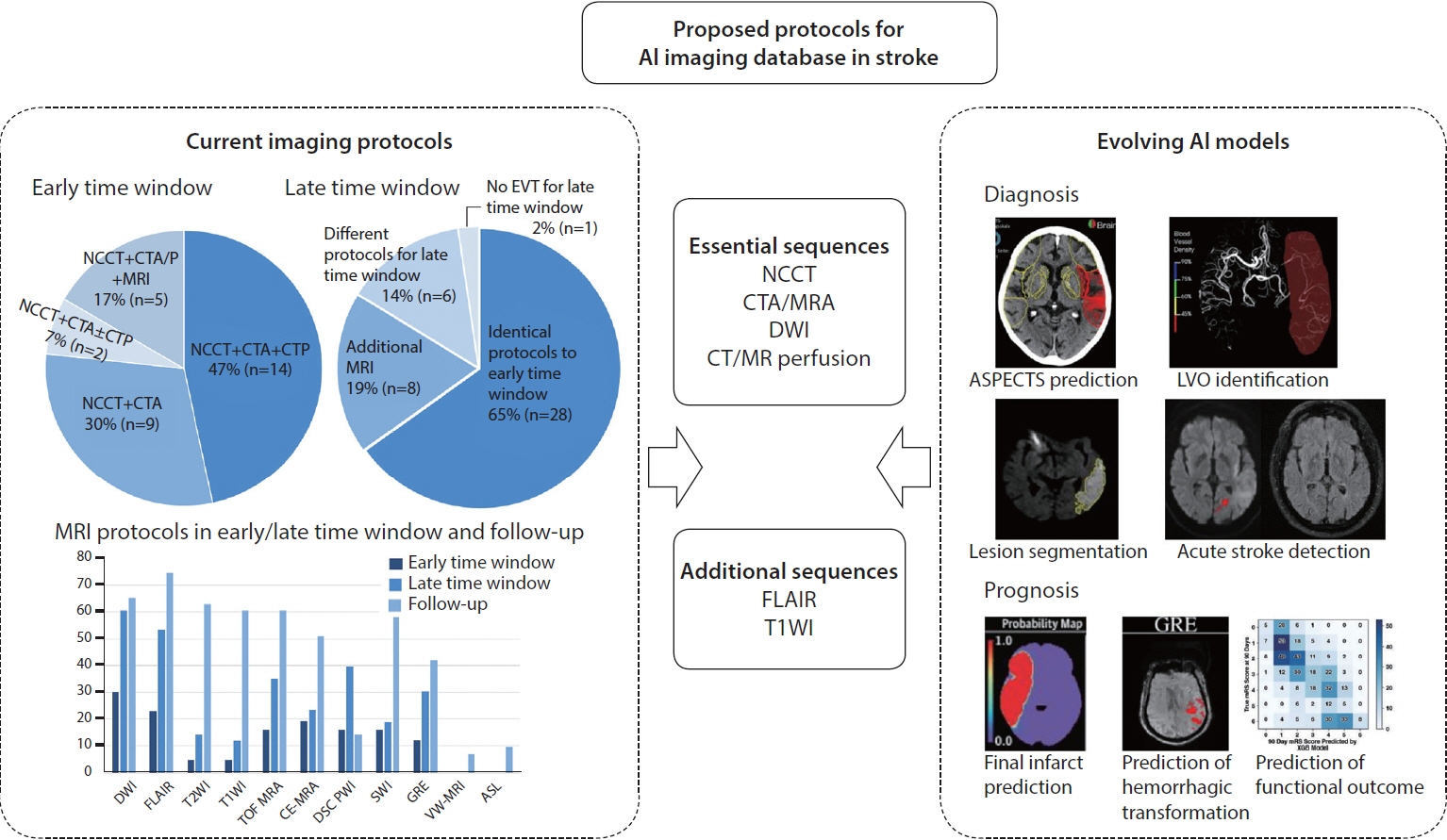
There was considerable heterogeneity in the imaging protocols for EVT candidates in the early and late time windows and posterior circulation stroke. Computed tomography (CT)-based protocols were adopted by 70% (30/43), and acquisition of noncontrast CT, CT angiography and CT perfusion in a single session was most commonly performed (47%, 14/30) with the preference of multiphase (70%, 21/30) over single phase CT angiography. More hospitals performed magnetic resonance imaging (MRI)-based protocols or additional MRI sequences in a late time window and posterior circulation stroke. Diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) were most commonly performed MRI sequences with considerable variation in performing other MRI sequences. AI models for diagnostic purposes required noncontrast CT, CT angiography and DWI while FLAIR, dynamic susceptibility contrast perfusion, and T1-weighted imaging (T1WI) were additionally required for prognostic AI models.
Conclusion
Given considerable heterogeneity in acute stroke imaging protocols at tertiary hospitals in South Korea, standardized and feasible imaging protocols are required for constructing AI database in acute stroke. The essential sequences may be noncontrast CT, DWI, CT/MR angiography and CT/MR perfusion while FLAIR and T1WI may be additionally required.
Figure
Reference
-
1. Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017; 69:2657–2664.2. Murray NM, Unberath M, Hager GD, Hui FK. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: a systematic review. J Neurointerv Surg. 2020; 12:156–164.
Article3. Mouridsen K, Thurner P, Zaharchuk G. Artificial intelligence applications in stroke. Stroke. 2020; 51:2573–2579.
Article4. Liebeskind DS, Malhotra K, Hinman JD. Imaging as the nidus of precision cerebrovascular health: a million brains initiative. JAMA Neurol. 2017; 74:257–258.
Article5. Kim B, You SH, Jung SC. A multicenter survey of acute stroke imaging protocols for endovascular thrombectomy. Neurointervention. 2021; 16:20–28.
Article6. Seo KD, Suh SH. Endovascular treatment in acute ischemic stroke: a nationwide survey in Korea. Neurointervention. 2018; 13:84–89.
Article7. Herweh C, Ringleb PA, Rauch G, Gerry S, Behrens L, Möhlenbruch M, et al. Performance of e-ASPECTS software in comparison to that of stroke physicians on assessing CT scans of acute ischemic stroke patients. Int J Stroke. 2016; 11:438–445.
Article8. van Os HJA, Ramos LA, Hilbert A, van Leeuwen M, van Walderveen MAA, Kruyt ND, MR CLEAN Registry Investigators, et al. Predicting outcome of endovascular treatment for acute ischemic stroke: potential value of machine learning algorithms. Front Neurol. 2018; 9:784.
Article9. Nagel S, Sinha D, Day D, Reith W, Chapot R, Papanagiotou P, et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int J Stroke. 2017; 12:615–622.
Article10. Guberina N, Dietrich U, Radbruch A, Goebel J, Deuschl C, Ringelstein A, et al. Detection of early infarction signs with machine learning-based diagnosis by means of the Alberta Stroke Program Early CT score (ASPECTS) in the clinical routine. Neuroradiology. 2018; 60:889–901.
Article11. Ho KC, Speier W, El-Saden S, Arnold CW. Classifying acute ischemic stroke onset time using deep imaging features. AMIA Annu Symp Proc. 2018; 2017:892–901.12. Lee H, Lee EJ, Ham S, Lee HB, Lee JS, Kwon SU, et al. Machine learning approach to identify stroke within 4.5 hours. Stroke. 2020; 51:860–866.
Article13. Amukotuwa SA, Straka M, Smith H, Chandra RV, Dehkharghani S, Fischbein NJ, et al. Automated detection of intracranial large vessel occlusions on computed tomography angiography: a single center experience. Stroke. 2019; 50:2790–2798.
Article14. Barreira C, Bouslama M, Lim J, Al-Bayati A, Saleem Y, Devlin T, et al. E-108 Aladin study: automated large artery occlusion detection in stroke imaging study – a multicenter analysis. J NeuroInterventional Surg. 2018; 10:A101–A102.15. Chen L, Bentley P, Rueckert D. Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. Neuroimage Clin. 2017; 15:633–643.
Article16. Woo I, Lee A, Jung SC, Lee H, Kim N, Cho SJ, et al. Fully automatic segmentation of acute ischemic lesions on diffusion-weighted imaging using convolutional neural networks: comparison with conventional algorithms. Korean J Radiol. 2019; 20:1275–1284.
Article17. Scherer M, Cordes J, Younsi A, Sahin YA, Götz M, Möhlenbruch M, et al. Development and validation of an automatic segmentation algorithm for quantification of intracerebral hemorrhage. Stroke. 2016; 47:2776–2782.
Article18. Maier O, Wilms M, von der Gablentz J, Krämer UM, Münte TF, Handels H. Extra tree forests for sub-acute ischemic stroke lesion segmentation in MR sequences. J Neurosci Methods. 2015; 240:89–100.
Article19. Pustina D, Coslett HB, Turkeltaub PE, Tustison N, Schwartz MF, Avants B. Automated segmentation of chronic stroke lesions using LINDA: lesion identification with neighborhood data analysis. Hum Brain Mapp. 2016; 37:1405–1421.
Article20. Bouslama M, Ravindran K, Harston G, Rodrigues GM, Pisani L, Haussen DC, et al. Noncontrast computed tomography e-stroke infarct volume is similar to RAPID computed tomography perfusion in estimating postreperfusion infarct volumes. Stroke. 2021; 52:634–641.
Article21. Mukherjee A, Muthusami P, Mohimen A, Srinivasan K, Babunath B, Sylaja P, et al. Noncontrast computed tomography versus computed tomography angiography source images for predicting final infarct size in anterior circulation acute ischemic stroke: a prospective cohort study. J Stroke Cerebrovasc Dis. 2017; 26:339–346.
Article22. Livne M, Boldsen JK, Mikkelsen IK, Fiebach JB, Sobesky J, Mouridsen K. Boosted tree model reforms multimodal magnetic resonance imaging infarct prediction in acute stroke. Stroke. 2018; 49:912–918.
Article23. Nielsen A, Hansen MB, Tietze A, Mouridsen K. Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. Stroke. 2018; 49:1394–1401.
Article24. Yu Y, Xie Y, Thamm T, Gong E, Ouyang J, Huang C, et al. Use of deep learning to predict final ischemic stroke lesions from initial magnetic resonance imaging. JAMA Netw Open. 2020; 3:e200772.
Article25. Yu Y, Guo D, Lou M, Liebeskind D, Scalzo F. Prediction of hemorrhagic transformation severity in acute stroke from source perfusion MRI. IEEE Trans Biomed Eng. 2018; 65:2058–2065.
Article26. Dharmasaroja P, Dharmasaroja PA. Prediction of intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke using multiple artificial neural networks. Neurol Res. 2012; 34:120–128.
Article27. Bentley P, Ganesalingam J, Carlton Jones AL, Mahady K, Epton S, Rinne P, et al. Prediction of stroke thrombolysis outcome using CT brain machine learning. Neuroimage Clin. 2014; 4:635–640.
Article28. Xie Y, Jiang B, Gong E, Li Y, Zhu G, Michel P, et al. Journal club: use of gradient boosting machine learning to predict patient outcome in acute ischemic stroke on the basis of imaging, demographic, and clinical information. AJR Am J Roentgenol. 2019; 212:44–51.
Article29. Bacchi S, Zerner T, Oakden-Rayner L, Kleinig T, Patel S, Jannes J. Deep learning in the prediction of ischaemic stroke thrombolysis functional outcomes: a pilot study. Acad Radiol. 2020; 27:e19–e23.30. Kim BJ, Kim YH, Kim N, Kwon SU, Kim SJ, Kim JS, et al. Lesion location-based prediction of visual field improvement after cerebral infarction. PLoS One. 2015; 10:e0143882.
Article31. Rondina JM, Filippone M, Girolami M, Ward NS. Decoding post-stroke motor function from structural brain imaging. Neuroimage Clin. 2016; 12:372–380.
Article32. Vilela P, Rowley HA. Brain ischemia: CT and MRI techniques in acute ischemic stroke. Eur J Radiol. 2017; 96:162–172.
Article33. Suh CH, Jung SC, Kim B, Cho SJ, Woo DC, Oh WY, et al. Neuroimaging in randomized, multi-center clinical trials of endovascular treatment for acute ischemic stroke: a systematic review. Korean J Radiol. 2020; 21:42–57.
Article34. Li B, Li H, Dong L, Huang G. Fast carotid artery MR angiography with compressed sensing based three-dimensional time-of-flight sequence. Magn Reson Imaging. 2017; 43:129–135.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep into the Brain: Artificial Intelligence in Stroke Imaging
- Application of Machine Learning and Deep Learning in Imaging of Ischemic Stroke
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging
- A Multicenter Survey of Acute Stroke Imaging Protocols for Endovascular Thrombectomy
- Commentary on An Application of Artificial Intelligence to Diagnostic Imaging of Spine Disease: Estimating Spinal Alignment From Moiré Images