Intest Res.
2023 Oct;21(4):471-480. 10.5217/ir.2023.00035.
Malnutrition and inflammation status in nonobese patients with inflammatory bowel disease are associated with nonalcoholic fatty liver disease: a retrospective study
- Affiliations
-
- 1Department of Gastroenterology and Medicine, Fukuoka University Faculty of Medicine, Fukuoka, Japan
- KMID: 2547197
- DOI: http://doi.org/10.5217/ir.2023.00035
Abstract
- Background/Aims
The frequency and details of nonalcoholic fatty liver disease (NAFLD) complications in patients with inflammatory bowel disease (IBD) remain unclear. This study aimed to clarify characteristics of NAFLD in patients with IBD.
Methods
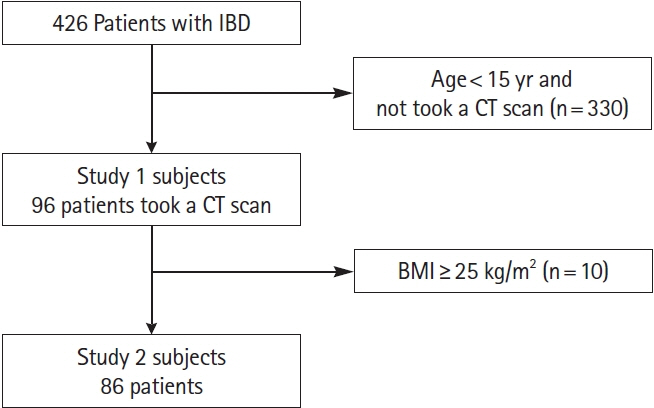
We retrospectively identified and enrolled patients with IBD diagnosed with or without NAFLD by undergoing abdominal computed tomography (CT) at our institution between 2005 and 2020. The primary endpoint was the complication rate of NAFLD in patients with IBD. Secondary endpoints were the clinical characteristics of nonobese patients with IBD and comorbid NAFLD and their association with nutritional and inflammatory parameters.
Results
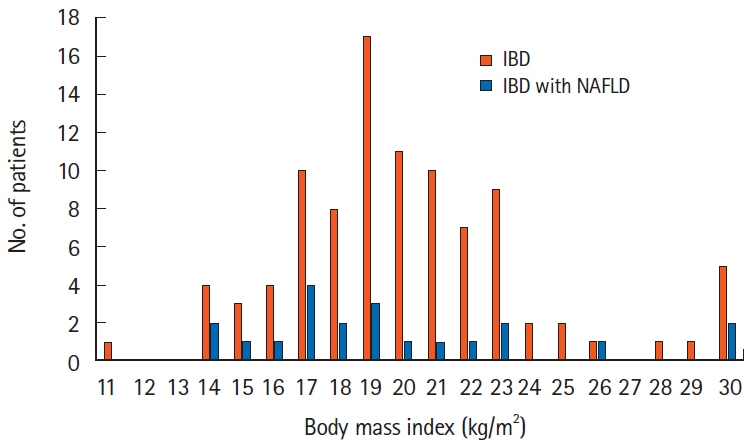
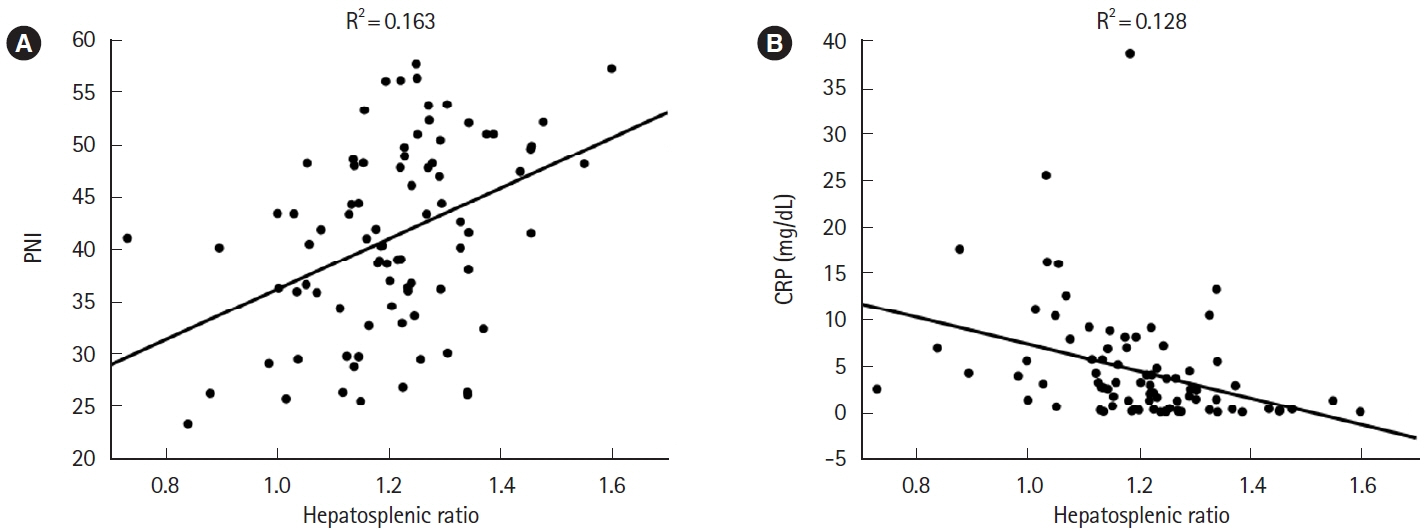
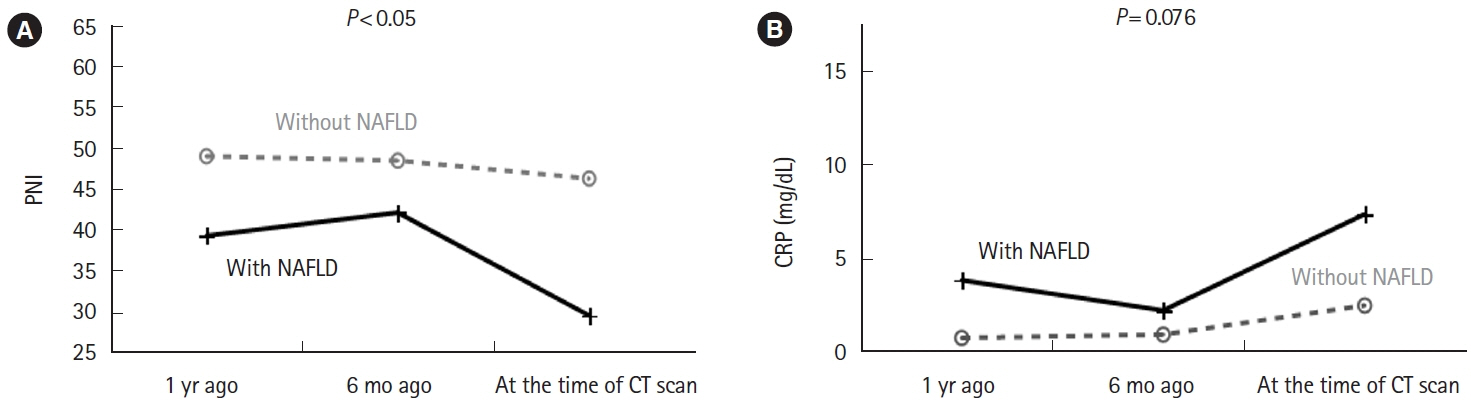
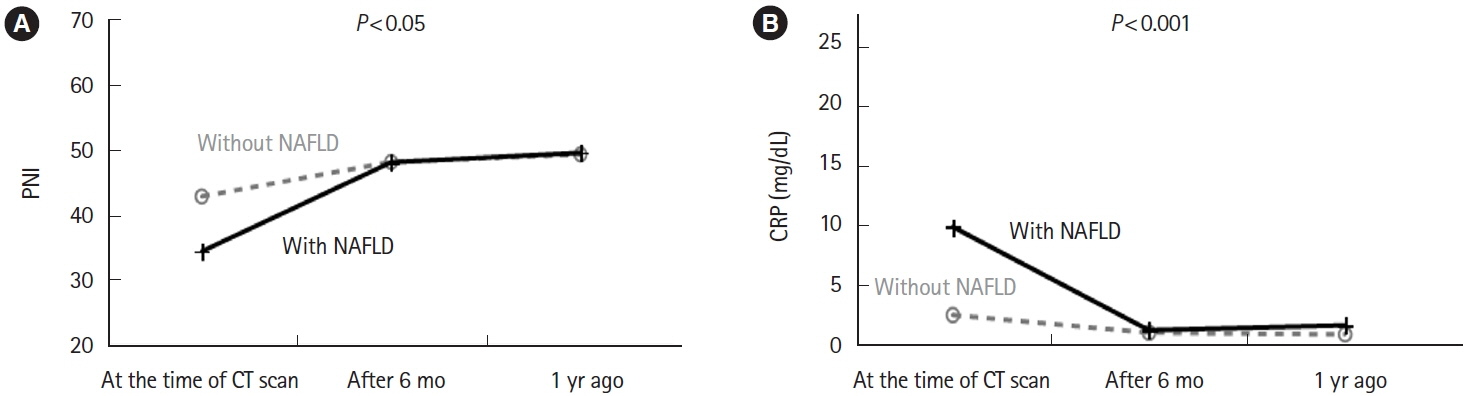
Twenty-one (21.9%) of 96 eligible patients with IBD also had NAFLD. In nonobese patients (defined as patients with a body mass index <25 kg/m2), C-reactive protein (CRP; P<0.001) and alanine aminotransferase (P=0.018) levels were higher and the albumin level (P=0.005) and prognostic nutritional index (PNI; P=0.002) values were lower in patients with NAFLD than in those without NAFLD. The PNI value was positively correlated (P<0.001) and the CRP level was negatively correlated (P=0.001) with the hepatosplenic ratio. However, in the NAFLD combined group, PNI (P<0.05) and CRP values (P<0.001) were improved over time after CT imaging by continuing IBD treatment.
Conclusions
Worsening nutritional and inflammatory status in IBD patients is associated with complications of NAFLD. Diagnosis of NAFLD in IBD patients using CT imaging might be useful not only for early detection of NAFLD but also in assessing the need for therapeutic intervention for IBD.
Figure
Reference
-
1. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020; 72:1605–1616.
Article2. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005; 143:722–728.
Article3. Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001; 121:91–100.
Article4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.5. Eguchi Y, Hyogo H, Ono M, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012; 47:586–595.
Article6. Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010; 51:1593–1602.
Article7. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017; 67:862–873.
Article8. Tobari M, Hashimoto E. Characteristic features of nonalcoholic fatty liver disease in Japan with a focus on the roles of age, sex and body mass index. Gut Liver. 2020; 14:537–545.
Article9. Wei JL, Leung JC, Loong TC, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015; 110:1306–1314.
Article10. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998; 115:182–205.
Article11. Kitchin B, Morgan S. Nutritional considerations in osteoporosis. Curr Opin Rheumatol. 2003; 15:476–480.
Article12. Gassull MA. Nutrition and inflammatory bowel disease: its relation to pathophysiology, outcome and therapy. Dig Dis. 2003; 21:220–227.
Article13. Pirlich M, Schütz T, Kemps M, et al. Prevalence of malnutrition in hospitalized medical patients: impact of underlying disease. Dig Dis. 2003; 21:245–251.
Article14. Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010; 21:315–319.
Article15. Gibiino G, Sartini A, Gitto S, et al. The other side of malnutrition in inflammatory bowel disease (IBD): non-alcoholic fatty liver disease. Nutrients. 2021; 13:2772.
Article16. Cappello M, Randazzo C, Bravatà I, et al. Liver function test abnormalities in patients with inflammatory bowel diseases: a hospital-based survey. Clin Med Insights Gastroenterol. 2014; 7:25–31.
Article17. Román AL, Muñoz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol. 2011; 17:2723–2733.
Article18. McGowan CE, Jones P, Long MD, Barritt AS 4th. Changing shape of disease: nonalcoholic fatty liver disease in Crohn’s disease-a case series and review of the literature. Inflamm Bowel Dis. 2012; 18:49–54.
Article19. Dundulis J, Helzberg J, Ansari S, El-Halawany H, Michelson R, Chhabra R. NAFLD appears to be the most common liver disease in IBD patients; IBD is likely a risk factor for NAFLD. Am J Gastroenterol. 2014; 109:S147.
Article20. Likhitsup A, Dundulis J, Ansari S, et al. Prevalence of non-alcoholic fatty liver disease on computed tomography in patients with inflammatory bowel disease visiting an emergency department. Ann Gastroenterol. 2019; 32:283–286.
Article21. Bargiggia S, Maconi G, Elli M, et al. Sonographic prevalence of liver steatosis and biliary tract stones in patients with inflammatory bowel disease: study of 511 subjects at a single center. J Clin Gastroenterol. 2003; 36:417–420.
Article22. Chao CY, Battat R, Al Khoury A, Restellini S, Sebastiani G, Bessissow T. Co-existence of non-alcoholic fatty liver disease and inflammatory bowel disease: a review article. World J Gastroenterol. 2016; 22:7727–7734.
Article23. Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G. Incidence and predictors of nonalcoholic fatty liver disease by serum biomarkers in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:1937–1944.
Article24. Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006; 130(2 Suppl 1):S70–S77.
Article25. Watanabe S, Hashimoto E, Ikejima K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015; 50:364–377.
Article26. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017; 377:2063–2072.
Article27. Adams LC, Lübbe F, Bressem K, Wagner M, Hamm B, Makowski MR. Non-alcoholic fatty liver disease in underweight patients with inflammatory bowel disease: a case-control study. PLoS One. 2018; 13:e0206450.
Article28. Examination Committee of Criteria for ‘Obesity Disease’ in Japan; Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002; 66:987–992.29. Mullie L, Afilalo J. CoreSlicer: a web toolkit for analytic morphomics. BMC Med Imaging. 2019; 19:15.
Article30. Hey P, Chew M, Wong D, et al. Moving computed tomography-based quantification of muscle mass to the mainstream: validation of a web-based platform to calculate skeletal muscle index in cirrhosis. Liver Transpl. 2022; 28:1944–1946.
Article31. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016; 46:951–963.
Article32. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980; 139:160–167.
Article33. Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017; 6:e004876.
Article34. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014; 140:1537–1549.
Article35. Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006; 40(Suppl 1):S34–S38.36. Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol. 2007; 189:W320–W323.
Article37. Flores A, Burstein E, Cipher DJ, Feagins LA. Obesity in inflammatory bowel disease: a marker of less severe disease. Dig Dis Sci. 2015; 60:2436–2445.
Article38. Gizard E, Ford AC, Bronowicki JP, Peyrin-Biroulet L. Systematic review: the epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2014; 40:3–15.
Article39. Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011; 141:1572–1585.
Article40. Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006; 81:1462–1471.
Article41. Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012; 28:310–313.42. Sagami S, Ueno Y, Tanaka S, et al. Significance of non-alcoholic fatty liver disease in Crohn’s disease: a retrospective cohort study. Hepatol Res. 2017; 47:872–881.
Article43. Domislović V, Knežević-Štromar I, Premužić M, et al. P140 Factors associated with development of NAFLD in patients with inflammatory bowel disease: a 5-year retrospective study on 225 patients. J Crohns Colitis. 2020; 14(Suppl_1):S207–S208.
Article44. Rodriguez-Duque JC, Calleja JL, Iruzubieta P, et al. Increased risk of MAFLD and liver fibrosis in inflammatory bowel disease independent of classic metabolic risk factors. Clin Gastroenterol Hepatol. 2023; 21:406–414.
Article45. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
Article46. Kim HY, Kim CW, Park CH, et al. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: the Fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int. 2016; 15:39–47.
Article47. Kim JA, Choi KM. Sarcopenia and fatty liver disease. Hepatol Int. 2019; 13:674–687.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Elucidating the association between nonalcoholic fatty liver disease and incidence of inflammatory bowel disease: a focus on systemic inflammation
- The Role of Inflammatory Mediators in the Pathogenesis of Nonalcoholic Fatty Liver Disease
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- The crosstalk between insulin resistance and nonalcoholic fatty liver disease/metabolic dysfunction-associated fatty liver disease: a culprit or a consequence?
- Nutrition and Chronic Liver Disease