Intest Res.
2023 Oct;21(4):460-470. 10.5217/ir.2022.00128.
Serum albumin is the strongest predictor of anti-tumor necrosis factor nonresponse in inflammatory bowel disease in resource-constrained regions lacking therapeutic drug monitoring
- Affiliations
-
- 1Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, India
- 4Department of Gastrointestinal Surgery, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2547196
- DOI: http://doi.org/10.5217/ir.2022.00128
Abstract
- Background/Aims
Evidence on predictors of primary nonresponse (PNR), and secondary loss of response (SLR) to anti-tumor necrosis factor (anti-TNF) agents in inflammatory bowel disease is scarce from Asia. We evaluated clinical/biochemical/molecular markers of PNR/SLR in ulcerative colitis (UC) and Crohn’s disease (CD).
Methods
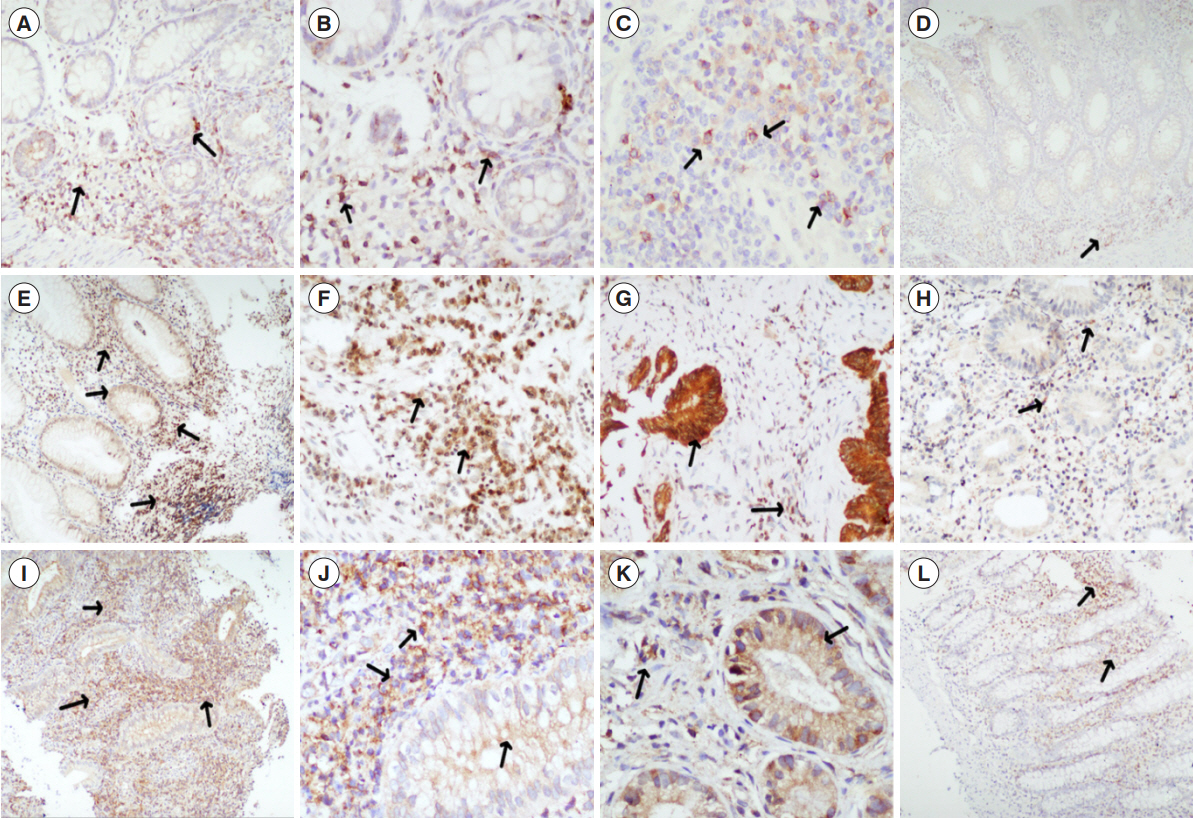
Inflammatory bowel disease patients treated with anti-TNF agents (January 2005–October 2020) were ambispectively included. Data concerning clinical and biochemical predictors was retrieved from a prospectively maintained database. Immunohistochemistry for expression of oncostatin M (OSM), OSM receptor (OSM-R), and interleukin-7 receptor (IL-7R) were done on pre anti-TNF initiation mucosal biopsies.
Results
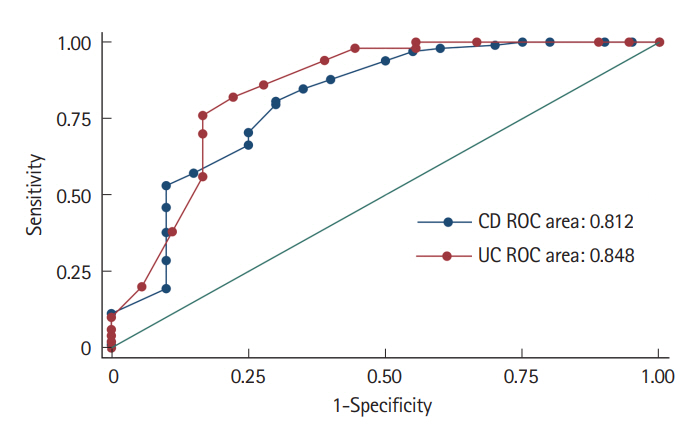
One-hundred eighty-six patients (118 CD, 68 UC: mean age, 34.1±13.7 years; median disease duration at anti-TNF initiation, 60 months; interquartile range, 28–100.5 months) were included. PNR was seen in 17% and 26.5% and SLR in 47% and 28% CD and UC patients, respectively. In CD, predictors of PNR were low albumin (P<0.001), postoperative recurrence (P=0.001) and high IL-7R expression (P<0.027) on univariate; and low albumin alone (hazard ratio [HR], 0.09; 95% confidence interval [CI], 0.03–0.28; P<0.001) on multivariate analysis respectively. Low albumin (HR, 0.31; 95% CI, 0.15–0.62; P=0.001) also predicted SLR. In UC, predictors of PNR were low albumin (P<0.001), and high C-reactive protein (P<0.001), OSM (P<0.04) and OSM-R (P=0.07) stromal expression on univariate; and low albumin alone (HR, 0.11; 95% CI, 0.03–0.39; P=0.001) on multivariate analysis respectively.
Conclusions
Low serum albumin at baseline significantly predicted PNR in UC and PNR/SLR in CD patients. Mucosal markers of PNR were high stromal OSM/OSM-R in UC and high IL-7R in CD patients.
Figure
Reference
-
1. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010; 4:355–366.
Article2. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article3. Arias MT, Vande Casteele N, Vermeire S, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:531–538.
Article4. Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020; 14:694–709.
Article5. West NR, Hegazy AN, Owens BM, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017; 23:579–589.6. Belarif L, Danger R, Kermarrec L, et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest. 2019; 129:1910–1925.
Article7. Ahuja V, Tandon RK. Inflammatory bowel disease in the AsiaPacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010; 11:134–147.
Article8. Kedia S, Ahuja V. Epidemiology of inflammatory bowel disease in India: the great shift East. Inflamm Intest Dis. 2017; 2:102–115.
Article9. Kumar P, Vuyyuru SK, Kante B, et al. Stringent screening strategy significantly reduces reactivation rates of tuberculosis in patients with inflammatory bowel disease on anti-TNF therapy in tuberculosis endemic region. Aliment Pharmacol Ther. 2022; 55:1431–1440.
Article10. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.
Article11. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010; 4:7–27.
Article12. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002; 122:512–530.
Article13. Kennedy NA, Heap GA, Green HD, et al. Predictors of antiTNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019; 4:341–353.14. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016; 7:e135.
Article15. Papamichael K, Rivals-Lerebours O, Billiet T, et al. Long-term outcome of patients with ulcerative colitis and primary nonresponse to infliximab. J Crohns Colitis. 2016; 10:1015–1023.
Article16. Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014; 8:129–136.
Article17. Peters CP, Eshuis EJ, Toxopeüs FM, et al. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014; 8:866–875.
Article18. Kathiresan S, Larson MG, Vasan RS, et al. Contribution of clinical correlates and 13 C-reactive protein gene polymorphisms to interindividual variability in serum C-reactive protein level. Circulation. 2006; 113:1415–1423.
Article19. Adams A, Gupta V, Mohsen W, et al. Early management of acute severe UC in the biologics era: development and international validation of a prognostic clinical index to predict steroid response. Gut. 2023; 72:433–442.20. Yang F, Bian C, Zhu L, Zhao G, Huang Z, Huang M. Effect of human serum albumin on drug metabolism: structural evidence of esterase activity of human serum albumin. J Struct Biol. 2007; 157:348–355.21. Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019; 43:181–193.
Article22. Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010; 48:297–308.
Article23. Kopylov U, Seidman E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Therap Adv Gastroenterol. 2016; 9:513–526.
Article24. Morita Y, Bamba S, Takahashi K, et al. Prediction of clinical and endoscopic responses to anti-tumor necrosis factor-α antibodies in ulcerative colitis. Scand J Gastroenterol. 2016; 51:934–941.
Article25. Hirai F, Takeda T, Takada Y, et al. Efficacy of enteral nutrition in patients with Crohn’s disease on maintenance anti-TNF-alpha antibody therapy: a meta-analysis. J Gastroenterol. 2020; 55:133–141.
Article26. Atreya R, Neurath MF. Mechanisms of molecular resistance and predictors of response to biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2018; 3:790–802.
Article27. Arora U, Ananthakrishnan AN, Kedia S, et al. Effect of oral tobacco use and smoking on outcomes of Crohn’s disease in India. J Gastroenterol Hepatol. 2018; 33:134–140.
Article28. Gupta A, Pratap Mouli V, Mohta S, et al. Antitubercular therapy given to differentiate Crohn’s disease from intestinal tuberculosis predisposes to stricture formation. J Crohns Colitis. 2020; 14:1611–1618.
Article29. Carrette F, Surh CD. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol. 2012; 24:209–217.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is the Therapeutic Drug Monitoring of Anti-TNF Agents Necessary in Korean Inflammatory Bowel Disease Patients?
- Biological Therapy for Inflammatory Bowel Disease in Children
- Stem Cells in Inflammatory Bowel Disease: New Potential Therapeutic Target
- Anti-tumor Necrosis Factor Agents and Tuberculosis in Inflammatory Bowel Disease
- Reversal of Immunogenicity in Pediatric Inflammatory Bowel Disease Patients Receiving Anti-Tumor Necrosis Factor Medications