Intest Res.
2023 Oct;21(4):452-459. 10.5217/ir.2022.00087.
Low prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease in India
- Affiliations
-
- 1Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, India
- 2Department of Internal Medicine, Dayanand Medical College and Hospital, Ludhiana, India
- 3Department of Pathology, Dayanand Medical College and Hospital, Ludhiana, India
- 4Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India
- 5P. D. Hinduja National Hospital and Medical Research Centre, Mumbai, India
- 6Research and Development Centre, Department of Gastroenterology, Dayanand Medical College and Hospital, Ludhiana, India
- 7Asian Institute of Gastroenterology, Hyderabad, India
- 8Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- KMID: 2547195
- DOI: http://doi.org/10.5217/ir.2022.00087
Abstract
- Background/Aims
Primary sclerosing cholangitis (PSC) represents the most common hepatobiliary extraintestinal manifestation of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). Limited data exist on PSC in patients with IBD from India. We aimed to assess the prevalence and disease spectrum of PSC in Indian patients with IBD.
Methods
Database of IBD patients at 5 tertiary care IBD centers in India were analyzed retrospectively. Data were extracted and the prevalence of PSC-IBD was calculated.
Results
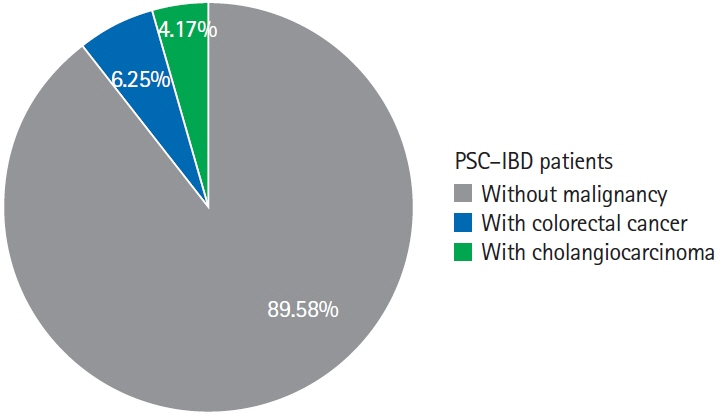
Forty-eight patients out of 12,216 patients with IBD (9,231 UC, 2,939 CD, and 46 IBD unclassified) were identified to have PSC, resulting in a prevalence of 0.39%. The UC to CD ratio was 7:1. Male sex and pancolitis (UC) or colonic CD were more commonly associated with PSC-IBD. The diagnosis of IBD preceded the diagnosis of PSC in most of the patients. Majority of the patients were symptomatic for liver disease at diagnosis. Eight patients (16.66%) developed cirrhosis, 5 patients (10.41%), all UC, developed malignancies (3 colorectal cancer [6.25%] and 2 cholangiocarcinoma [4.16%]), and 3 patients died (2 decompensated liver disease [4.16%] and 1 cholangiocarcinoma [2.08%]) on follow-up. None of the patients mandated surgical therapy for IBD.
Conclusions
Concomitant PSC in patients with IBD is uncommon in India and is associated with lower rates of development of malignancies.
Keyword
Figure
Cited by 2 articles
-
Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
You Sun Kim, Edward H. Hurley, Yoojeong Park, Sungjin Ko
Intest Res. 2023;21(4):420-432. doi: 10.5217/ir.2023.00039.Regional variations in the prevalence of primary sclerosing cholangitis associated with inflammatory bowel disease
Kwang Woo Kim, Hyoun Woo Kang
Intest Res. 2023;21(4):413-414. doi: 10.5217/ir.2023.00133.
Reference
-
1. Smith MP, Loe RH. Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am J Surg. 1965; 110:239–246.2. Lazaridis KN, LaRusso NF. Primary sclerosing cholangitis. N Engl J Med. 2016; 375:1161–1170.
Article3. de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015; 21:1956–1971.
Article4. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Hepatobiliary and coexisting pancreatic duct abnormalities in patients with inflammatory bowel disease. Scand J Gastroenterol. 1997; 32:153–161.
Article5. Duboc H, Rajca S, Rainteau D, et al. Connecting dysbiosis, bileacid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013; 62:531–539.
Article6. Quraishi MN, Sergeant M, Kay G, et al. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut. 2017; 66:386–388.
Article7. Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002; 359:150–157.
Article8. Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012; 491:119–124.9. Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis: aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008; 48 Suppl 1:S38–S57.10. Loftus EV Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005; 54:91–96.
Article11. Sinakos E, Samuel S, Enders F, Loftus EV Jr, Sandborn WJ, Lindor KD. Inflammatory bowel disease in primary sclerosing cholangitis: a robust yet changing relationship. Inflamm Bowel Dis. 2013; 19:1004–1009.12. Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory bowel disease and primary sclerosing cholangitis: a review of the phenotype and associated specific features. Gut Liver. 2018; 12:17–29.
Article13. Beheshti-Maal A, Tamimi A, Iravani S, et al. PSC associated inflammatory bowel disease: a distinct entity. Expert Rev Gastroenterol Hepatol. 2022; 16:129–139.
Article14. Lindström L, Lapidus A, Ost A, Bergquist A. Increased risk of colorectal cancer and dysplasia in patients with Crohn’s colitis and primary sclerosing cholangitis. Dis Colon Rectum. 2011; 54:1392–1397.
Article15. Claessen MM, Lutgens MW, van Buuren HR, et al. More rightsided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis. 2009; 15:1331–1336.16. Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997; 41:522–525.
Article17. Wijnands AM, de Jong ME, Lutgens MW, et al. Prognostic factors for advanced colorectal neoplasia in inflammatory bowel disease: systematic review and meta-analysis. Gastroenterology. 2021; 160:1584–1598.
Article18. Barberio B, Massimi D, Cazzagon N, Zingone F, Ford AC, Savarino EV. Prevalence of primary sclerosing cholangitis in patients with inflammatory bowel disease: a systematic review and meta-analysis. Gastroenterology. 2021; 161:1865–1877.
Article19. Singh B, Kedia S, Konijeti G, et al. Extraintestinal manifestations of inflammatory bowel disease and intestinal tuberculosis: frequency and relation with disease phenotype. Indian J Gastroenterol. 2015; 34:43–50.
Article20. Banerjee R, Pal P, Hilmi I, et al. Emerging inflammatory bowel disease demographics, phenotype, and treatment in South Asia, South-East Asia, and Middle East: preliminary findings from the inflammatory bowel disease-emerging nations’ consortium. J Gastroenterol Hepatol. 2022; 37:1004–1015.
Article21. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article22. Chapman MH, Thorburn D, Hirschfield GM, et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019; 68:1356–1378.
Article23. Guerra I, Bujanda L, Castro J, et al. Clinical characteristics, associated malignancies and management of primary sclerosing cholangitis in inflammatory bowel disease patients: a multicentre retrospective cohort study. J Crohns Colitis. 2019; 13:1492–1500.24. Lunder AK, Hov JR, Borthne A, et al. Prevalence of sclerosing cholangitis detected by magnetic resonance cholangiography in patients with long-term inflammatory bowel disease. Gastroenterology. 2016; 151:660–669. e4.
Article25. Banerjee R, Pal P, Nugent Z, et al. IBD in India: similar phenotype but different demographics than the west. J Clin Gastroenterol. 2020; 54:725–732.26. Olsson R, Danielsson A, Järnerot G, et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991; 100(5 Pt 1):1319–1323.
Article27. Wewer V, Gluud C, Schlichting P, Burcharth F, Binder V. Prevalence of hepatobiliary dysfunction in a regional group of patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1991; 26:97–102.
Article28. Roberts H, Rai SN, Pan J, et al. Extraintestinal manifestations of inflammatory bowel disease and the influence of smoking. Digestion. 2014; 90:122–129.
Article29. Wijarnpreecha K, Panjawatanan P, Mousa OY, Cheungpasitporn W, Pungpapong S, Ungprasert P. Association between smoking and risk of primary sclerosing cholangitis: a systematic review and meta-analysis. United European Gastroenterol J. 2018; 6:500–508.
Article30. Kaplan GG, Laupland KB, Butzner D, Urbanski SJ, Lee SS. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007; 102:1042–1049.
Article31. Banerjee R, Pal P, Mak JW, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resourcelimited settings in Asia. Lancet Gastroenterol Hepatol. 2020; 5:1076–1088.
Article32. Halliday JS, Djordjevic J, Lust M, et al. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn’s disease. J Crohns Colitis. 2012; 6:174–181.
Article33. Navaneethan U, Venkatesh PG, Jegadeesan R, et al. Comparison of outcomes for patients with primary sclerosing cholangitis associated with ulcerative colitis and Crohn’s disease. Gastroenterol Rep (Oxf). 2016; 4:43–49.
Article34. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014; 383:2168–2179.
Article35. Björnsson E, Angulo P. Cholangiocarcinoma in young individuals with and without primary sclerosing cholangitis. Am J Gastroenterol. 2007; 102:1677–1682.
Article36. Bopanna S, Kedia S, Das P, et al. Long-term follow-up reveals high incidence of colorectal cancer in Indian patients with inflammatory bowel disease. United European Gastroenterol J. 2017; 5:708–714.
Article37. Desai D, Shah S, Deshmukh A, et al. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J Gastroenterol. 2015; 21:3644–3649.
Article38. Bopanna S, Roy M, Das P, et al. Role of random biopsies in surveillance of dysplasia in ulcerative colitis patients with high risk of colorectal cancer. Intest Res. 2016; 14:264–269.
Article39. Karlsen TH. Primary sclerosing cholangitis: 50 years of a gutliver relationship and still no love? Gut. 2016; 65:1579–1581.
Article40. Ananthakrishnan AN, Cagan A, Gainer VS, et al. Mortality and extraintestinal cancers in patients with primary sclerosing cholangitis and inflammatory bowel disease. J Crohns Colitis. 2014; 8:956–963.
Article41. Hill MJ. Bile flow and colon cancer. Mutat Res. 1990; 238:313–320.
Article42. Farhana L, Nangia-Makker P, Arbit E, et al. Bile acid: a potential inducer of colon cancer stem cells. Stem Cell Res Ther. 2016; 7:181.
Article43. Garrett WS. The gut microbiota and colon cancer. Science. 2019; 364:1133–1135.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biliary Tract & Pancreas; A Case of Cholangiocarcinoma Suggested as Developing in the Patient with Primary Sclerosing Cholangitis
- A novel clinical trial for primary sclerosing cholangitis from Asia: All regional endeavors should improve global management of primary sclerosing cholangitis: Editorial on “Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial”
- A Case of Chronic Lymphoplasmacellular Osteomyelitiswith Autoimmune Hepatitis/Primary SclerosingCholangitis Overlap Syndrome in a Child
- Hepatobiliary Manifestation of Inflammatory Bowel Disease
- Primary Sclerosing Cholangitis in Patients with Ulcerative Colitis: Two Case Reports