J Rheum Dis.
2023 Oct;30(4):243-250. 10.4078/jrd.2023.0024.
Elevated BMPR2 expression amplifies osteoblast differentiation in ankylosing spondylitis
- Affiliations
-
- 1Hanyang University Institute for Rheumatology Research (HYIRR), Seoul, Korea
- 2Department of Translational Medicine Science, Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- 3Department of Rheumatology, Chonnam National University Medical School and Hospital, Gwangju, Korea
- 4Department of Orthopedic Surgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 5Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- KMID: 2546240
- DOI: http://doi.org/10.4078/jrd.2023.0024
Abstract
Objective
Bone morphogenetic protein receptor type 2 (BMPR2) has been associated with radiographic changes in ankylosing spondylitis (AS), but further characterization of the cellular signaling pathway in osteoprogenitor (OP) is not clearly understood. The aim of this study was to investigate the expression of BMPR2 and bone morphogenetic protein 2 (BMP2)-mediated responsibility in AS.
Methods
We collected 10 healthy control (HC) and 14 AS-OPs derived from facet joints. Subsequently, we then conducted RNA sequencing with two samples per group and selected BMP-related genes. Facet joint tissues and derived primary OPs were evaluated by validation of selected RNA sequencing data, immunohistochemistry, and comparison of osteogenic differentiation potential.
Results
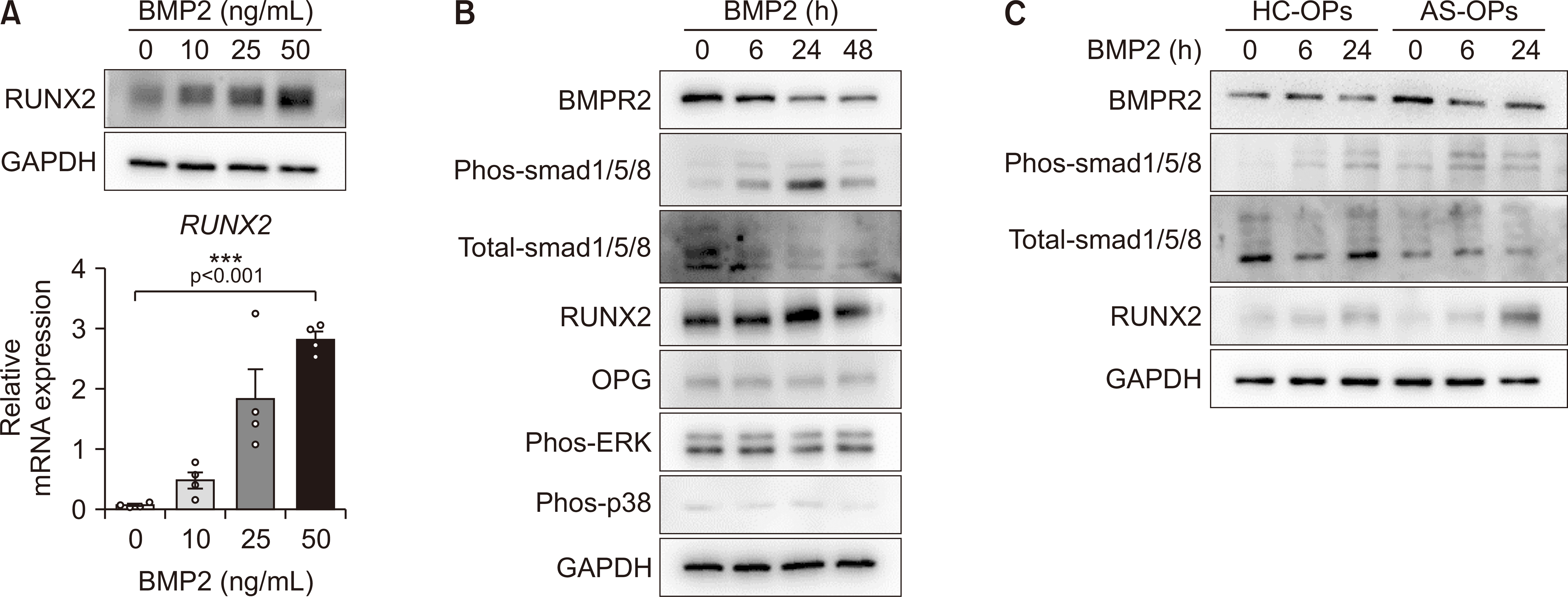
Based on RNA-sequencing analysis, we found that BMPR2 expression is higher in AS-OPs compared to in HC-OPs. We also validated the increased BMPR2 expression in facet joint tissues with AS and its derived OPs in messenger RNA and protein levels. Additionally, primary AS-OPs showed much greater response to osteogenic differentiation induced by BMP2 and a higher capacity for smad1/5/8-induced RUNX2 expression compared to HCs.
Conclusion
The expression of BMPR2 was found to be significantly increased in facet joint tissues of patients with AS. These findings suggest that BMPR2 may play a role in the BMP2-mediated progression of AS.
Keyword
Figure
Reference
-
1. Mauro D, Thomas R, Guggino G, Lories R, Brown MA, Ciccia F. 2021; Ankylosing spondylitis: an autoimmune or autoinflammatory disease? Nat Rev Rheumatol. 17:387–404. DOI: 10.1038/s41584-021-00625-y. PMID: 34113018.
Article2. Inman RD. 2021; Axial spondyloarthritis: current advances, future challenges. J Rheum Dis. 28:55–9. DOI: 10.4078/jrd.2021.28.2.55. PMID: 37476012. PMCID: PMC10324891.
Article3. Deodhar A. 2018; Spondyloarthropathies: TNF inhibitors and structural damage in ankylosing spondylitis. Nat Rev Rheumatol. 14:5–6. DOI: 10.1038/nrrheum.2017.197. PMID: 29213122.
Article4. Karim MS, Madamanchi A, Dutko JA, Mullins MC, Umulis DM. 2021; Heterodimer-heterotetramer formation mediates enhanced sensor activity in a biophysical model for BMP signaling. PLoS Comput Biol. 17:e1009422. DOI: 10.1371/journal.pcbi.1009422. PMID: 34591841. PMCID: PMC8509922.
Article5. Carter S, Braem K, Lories RJ. 2012; The role of bone morphogenetic proteins in ankylosing spondylitis. Ther Adv Musculoskelet Dis. 4:293–9. DOI: 10.1177/1759720X12444175. PMID: 22859928. PMCID: PMC3403253.
Article6. Lories RJ, Derese I, Luyten FP. 2005; Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 115:1571–9. DOI: 10.1172/JCI23738. PMID: 15902307. PMCID: PMC1090472.
Article7. Ding L, Yin Y, Hou Y, Jiang H, Zhang J, Dai Z, et al. 2021; microRNA-214-3p suppresses ankylosing spondylitis fibroblast osteogenesis via BMP-TGFβ axis and BMP2. Front Endocrinol (Lausanne). 11:609753. DOI: 10.3389/fendo.2020.609753. PMID: 33935961. PMCID: PMC8082363.
Article8. Chen HA, Chen CH, Lin YJ, Chen PC, Chen WS, Lu CL, et al. 2010; Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 37:2126–32. DOI: 10.3899/jrheum.100200. PMID: 20682677.
Article9. Qi D, Tian X, Wang Y, Zheng G, Zhang X. 2020; BMP2 variants in the risk of ankylosing spondylitis. J Cell Biochem. 121:3935–40. DOI: 10.1002/jcb.29563. PMID: 31713925.
Article10. Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, et al. 2016; Imbalance between bone morphogenetic protein 2 and Noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 68:430–40. DOI: 10.1002/art.39433. PMID: 26413886.
Article11. Liu Z, Wang P, Cen S, Gao L, Xie Z, Wu X, et al. 2019; Increased BMPR1A expression enhances the adipogenic differentiation of mesenchymal stem cells in patients with ankylosing spondylitis. Stem Cells Int. 2019:4143167. DOI: 10.1155/2019/4143167. PMID: 31827527. PMCID: PMC6885782.
Article12. Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, et al. 2015; Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 74:1387–93. DOI: 10.1136/annrheumdis-2013-204835. PMID: 24651623. PMCID: PMC4470170.
Article13. Skillington J, Choy L, Derynck R. 2002; Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 159:135–46. DOI: 10.1083/jcb.200204060. PMID: 12379805. PMCID: PMC2173483.
Article14. van der Linden S, Valkenburg HA, Cats A. 1984; Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27:361–8. DOI: 10.1002/art.1780270401. PMID: 6231933.15. Jo S, Lee JS, Nam B, Lee YL, Kim H, Lee EY, et al. 2022; SOX9+ enthesis cells are associated with spinal ankylosis in ankylosing spondylitis. Osteoarthritis Cartilage. 30:280–90. DOI: 10.1016/j.joca.2021.11.013. PMID: 34826571.
Article16. Jo S, Lee SH, Park J, Nam B, Kim H, Youn J, et al. 2023; Platelet-derived growth factor B is a key element in the pathological bone formation of ankylosing spondylitis. J Bone Miner Res. 38:300–12. DOI: 10.1002/jbmr.4751. PMID: 36422470.
Article17. Park PR, Jo S, Jin SH, Kim TJ. 2020; MicroRNA-10b plays a role in bone formation by suppressing interleukin-22 in ankylosing spondylitis. J Rheum Dis. 27:61–7. DOI: 10.4078/jrd.2020.27.1.61.
Article18. Jo S, Han J, Lee YL, Yoon S, Lee J, Wang SE, et al. 2019; Regulation of osteoblasts by alkaline phosphatase in ankylosing spondylitis. Int J Rheum Dis. 22:252–61. DOI: 10.1111/1756-185X.13419. PMID: 30415492.
Article19. Jo S, Kang S, Han J, Choi SH, Park YS, Sung IH, et al. 2018; Accelerated osteogenic differentiation of human bone-derived cells in ankylosing spondylitis. J Bone Miner Metab. 36:307–13. DOI: 10.1007/s00774-017-0846-3. PMID: 28589411.
Article20. Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. 2018; IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 20:115. DOI: 10.1186/s13075-018-1582-3. PMID: 29880011. PMCID: PMC5992730.
Article21. Zheng G, Xie Z, Wang P, Li J, Li M, Cen S, et al. 2019; Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment. Cell Death Dis. 10:350. DOI: 10.1038/s41419-019-1586-1. PMID: 31024000. PMCID: PMC6484086.
Article22. Koo BS, Oh JS, Park SY, Shin JH, Ahn GY, Lee S, et al. 2020; Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis. 79:1327–32. DOI: 10.1136/annrheumdis-2019-216741. PMID: 32660979.
Article23. Lim KE, Park NR, Che X, Han MS, Jeong JH, Kim SY, et al. 2015; Core binding factor β of osteoblasts maintains cortical bone mass via stabilization of Runx2 in mice. J Bone Miner Res. 30:715–22. DOI: 10.1002/jbmr.2397. PMID: 25358268. PMCID: PMC7363154.
Article24. Gomez-Puerto MC, Iyengar PV, García de Vinuesa A, Ten Dijke P, Sanchez-Duffhues G. 2019; Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 247:9–20. DOI: 10.1002/path.5170. PMID: 30246251. PMCID: PMC6587955.25. Kim WJ, Shin HL, Kim BS, Kim HJ, Ryoo HM. 2020; RUNX2-modifying enzymes: therapeutic targets for bone diseases. Exp Mol Med. 52:1178–84. DOI: 10.1038/s12276-020-0471-4. PMID: 32788656. PMCID: PMC8080656.
Article26. Croes M, Kruyt MC, Groen WM, van Dorenmalen KMA, Dhert WJA, Öner FC, et al. 2018; Interleukin 17 enhances bone morphogenetic protein-2-induced ectopic bone formation. Sci Rep. 8:7269. DOI: 10.1038/s41598-018-25564-9. PMID: 29740080. PMCID: PMC5940874.
Article27. Park MC, Park YB, Lee SK. 2008; Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 37:200–4. DOI: 10.1080/03009740701774941. PMID: 18465455.
Article28. Lories RJ, Derese I, Ceuppens JL, Luyten FP. 2003; Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast-like synoviocyte apoptosis. Arthritis Rheum. 48:2807–18. DOI: 10.1002/art.11389. PMID: 14558086.
Article29. Briolay A, El Jamal A, Arnolfo P, Le Goff B, Blanchard F, Magne D, et al. 2020; Enhanced BMP-2/BMP-4 ratio in patients with peripheral spondyloarthritis and in cytokine- and stretch-stimulated mouse chondrocytes. Arthritis Res Ther. 22:234. DOI: 10.1186/s13075-020-02330-9. PMID: 33046134. PMCID: PMC7552569.
Article30. Grandon B, Rincheval-Arnold A, Jah N, Corsi JM, Araujo LM, Glatigny S, et al. 2019; HLA-B27 alters BMP/TGFβ signalling in Drosophila, revealing putative pathogenic mechanism for spondyloarthritis. Ann Rheum Dis. 78:1653–62. DOI: 10.1136/annrheumdis-2019-215832. PMID: 31563893.
Article31. Jah N, Jobart-Malfait A, Ermoza K, Noteuil A, Chiocchia G, Breban M, et al. 2020; HLA-B27 subtypes predisposing to ankylosing spondylitis accumulate in an endoplasmic reticulum-derived compartment apart from the peptide-loading complex. Arthritis Rheumatol. 72:1534–46. DOI: 10.1002/art.41281. PMID: 32270915.32. Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, et al. 2008; BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 14:1363–9. Erratum in: Nat Med 2009;15:117. DOI: 10.1038/nm.1888. PMID: 19029982. PMCID: PMC2846458.33. Breban M, Glatigny S, Cherqaoui B, Beaufrère M, Lauraine M, Rincheval-Arnold A, et al. 2021; Lessons on SpA pathogenesis from animal models. Semin Immunopathol. 43:207–19. DOI: 10.1007/s00281-020-00832-x. PMID: 33449154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ankylosing Spondylitis: Prevention And Surgical Correction Of Deformity
- CT Evaluation of Sacroiliitis' Differentiation of Infectious Sacroiliitis versus Ankylosing Spondylitis
- Treatment of Cervical Cord Injury with Ankylosing Spondylitis: Case Report
- Orthopaedic Management of Ankylosing Spondylitis
- Clinieal Values of Single Photon Emission Computed Tomography ( SPECT ) in Ankylosing Spondylitis