Diabetes Metab J.
2023 Sep;47(5):703-714. 10.4093/dmj.2022.0205.
Glucose Regulation after Partial Pancreatectomy: A Comparison of Pancreaticoduodenectomy and Distal Pancreatectomy in the Short and Long Term
- Affiliations
-
- 1Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2546128
- DOI: http://doi.org/10.4093/dmj.2022.0205
Abstract
- Background
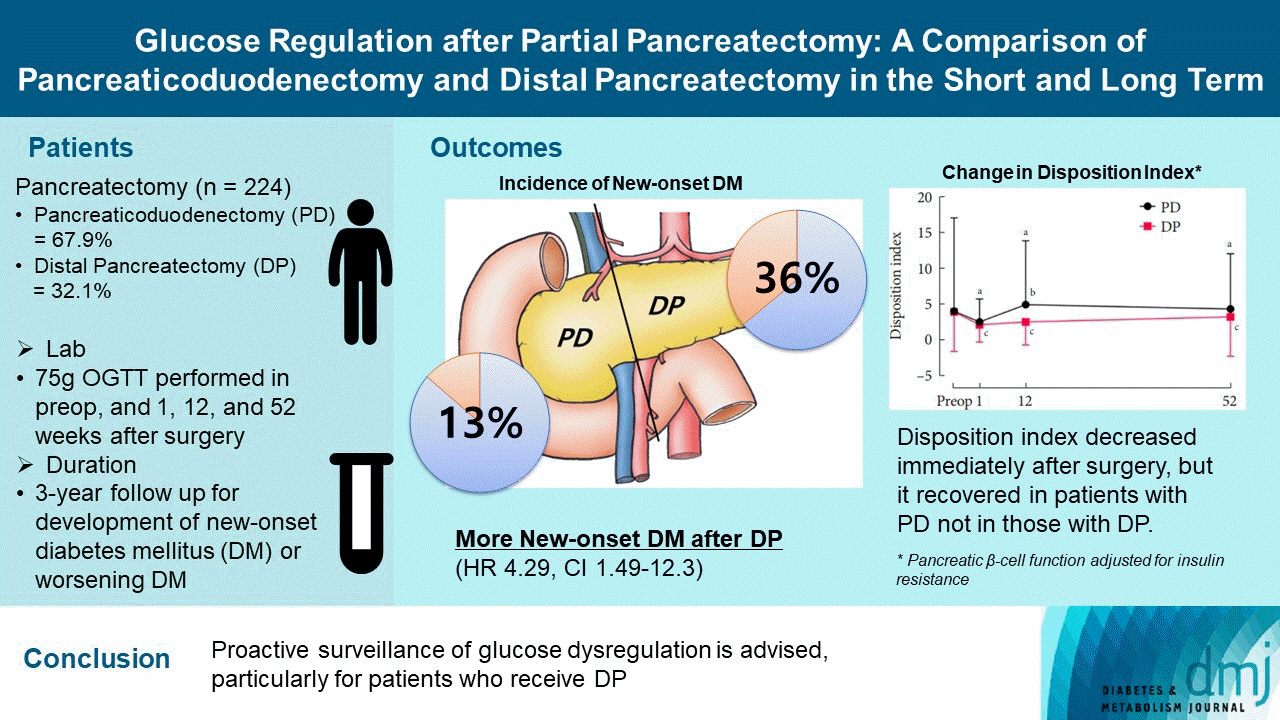
Long term quality of life is becoming increasingly crucial as survival following partial pancreatectomy rises. The purpose of this study was to investigate the difference in glucose dysregulation after pancreaticoduodenectomy (PD) or distal pancreatectomy (DP).
Methods
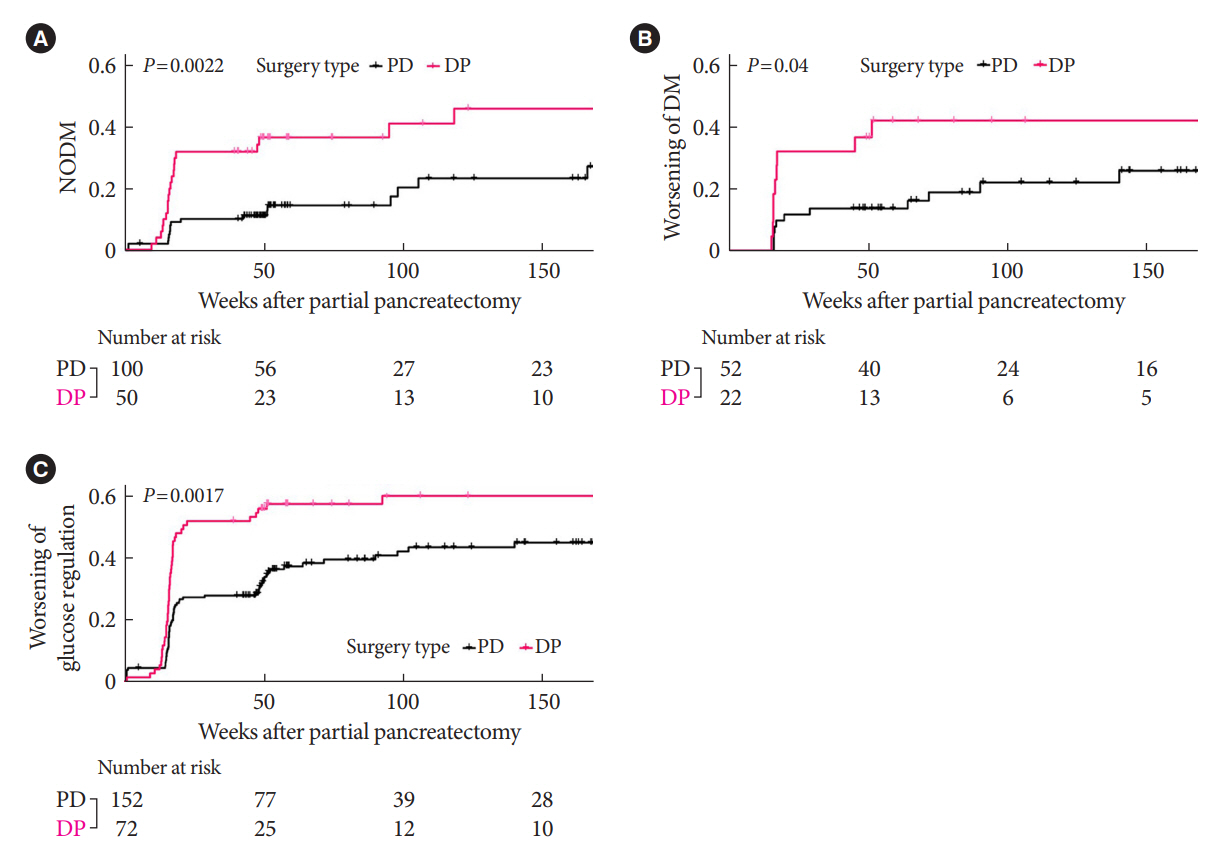
In this prospective observational study from 2015 to 2018, 224 patients who underwent partial pancreatectomy were selected: 152 (67.9%) received PD and 72 (32.1%) received DP. Comprehensive assessment for glucose regulation, including a 75 g oral glucose tolerance test was conducted preoperatively, and 1, 12, and 52 weeks after surgery. Patients were further monitored up to 3 years to investigate development of new-onset diabetes mellitus (NODM) in patients without diabetes mellitus (DM) at baseline or worsening of glucose regulation (≥1% increase in glycosylated hemoglobin [HbA1c]) in those with preexisting DM.
Results
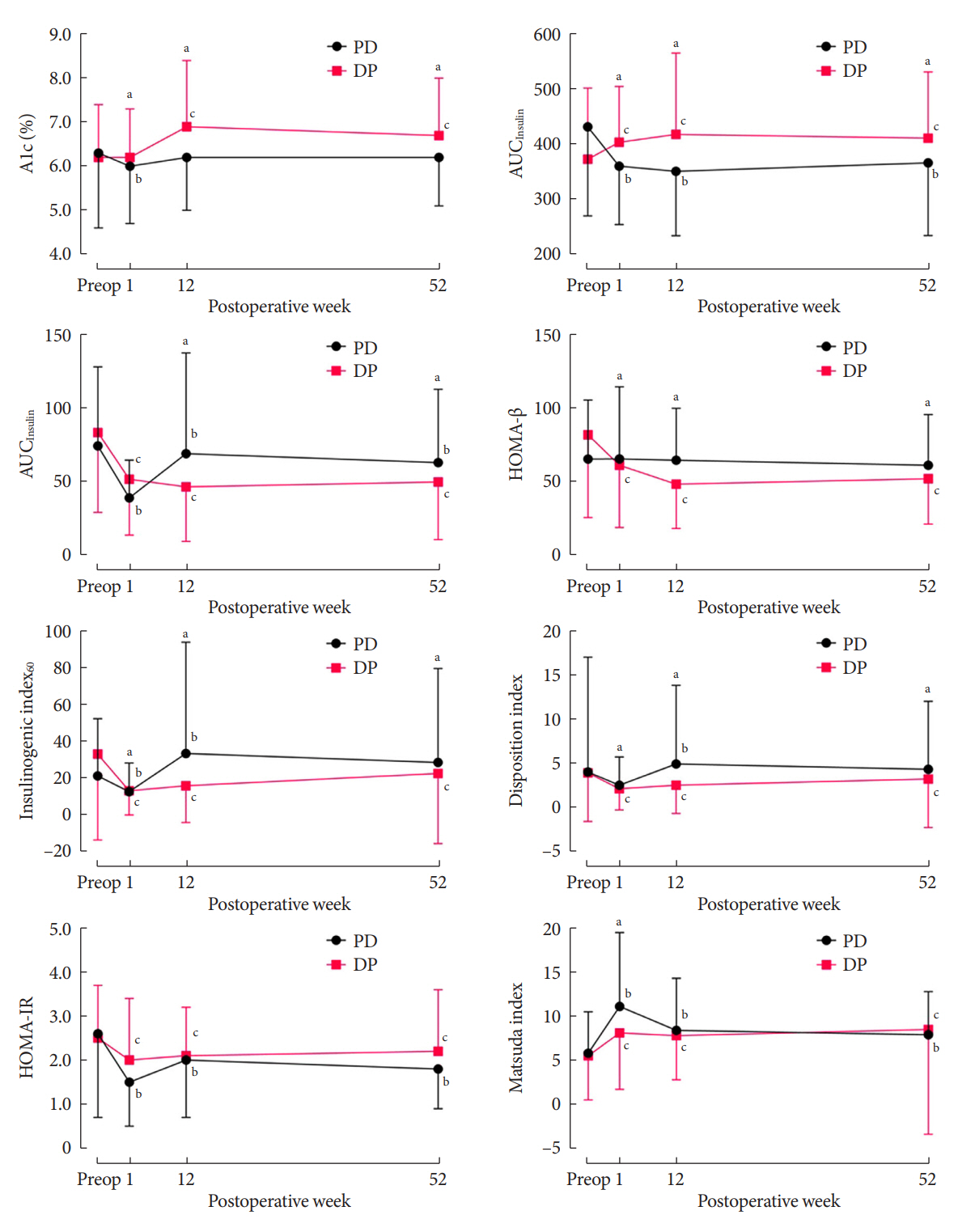
The disposition index, an integrated measure of β-cell function, decreased 1 week after surgery in both groups, but it increased more than baseline level in the PD group while its decreased level was maintained in the DP group, resulting in a between-group difference at the 1-year examination (P<0.001). During follow-up, the DP group showed higher incidence of NODM and worsening of glucose regulation than the PD group with hazard ratio (HR) 4.29 (95% confidence interval [CI], 1.49 to 12.3) and HR 2.15 (95% CI, 1.09 to 4.24), respectively, in the multivariate analysis including dynamic glycemic excursion profile. In the DP procedure, distal DP and spleen preservation were associated with better glucose regulation. DP had a stronger association with glucose dysregulation than PD.
Conclusion
Proactive surveillance of glucose dysregulation is advised, particularly for patients who receive DP.
Figure
Reference
-
1. Zhang Q, Zeng L, Chen Y, Lian G, Qian C, Chen S, et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract. 2016; 2016:8962321.2. Feng Q, Xin Z, Zhu B, Liao M, Liao W, Zeng Y. Perioperative and short-term oncological outcomes following laparoscopic versus open pancreaticoduodenectomy after learning curve in the past 10 years: a systematic review and meta-analysis. Gland Surg. 2021; 10:1655–68.3. Lyu Y, Cheng Y, Wang B, Zhao S, Chen L. Assessment of laparoscopic versus open distal pancreatectomy: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol. 2022; 31:350–8.4. Kim K, Yu JI, Jung W, Kim TH, Seong J, Kim WC, et al. Role of adjuvant radiotherapy in extrahepatic bile duct cancer: a multicenter retrospective study (Korean Radiation Oncology Group 18-14). Eur J Cancer. 2021; 157:31–9.5. Torphy RJ, Fujiwara Y, Schulick RD. Pancreatic cancer treatment: better, but a long way to go. Surg Today. 2020; 50:1117–25.6. Lee M, Kang JS, Kim H, Kwon W, Lee SH, Ryu JK, et al. Impact of conversion surgery on survival in locally advanced pancreatic cancer patients treated with FOLFIRINOX chemotherapy. J Hepatobiliary Pancreat Sci. 2023; 30:111–21.7. Shaw K, Thomas AS, Rosario V, Kwon W, Schrope BA, Sugahara K, et al. Long term quality of life amongst pancreatectomy patients with diabetes mellitus. Pancreatology. 2021; 21:501–8.8. Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011; 11:279–94.9. Maeda H, Hanazaki K. Pancreatogenic diabetes after pancreatic resection. Pancreatology. 2011; 11:268–76.10. Wu L, Nahm CB, Jamieson NB, Samra J, Clifton-Bligh R, Mittal A, et al. Risk factors for development of diabetes mellitus (type 3c) after partial pancreatectomy: a systematic review. Clin Endocrinol (Oxf). 2020; 92:396–406.11. Maxwell DW, Jajja MR, Galindo RJ, Zhang C, Nadeem SO, Sweeney JF, et al. Post-pancreatectomy diabetes index: a validated score predicting diabetes development after major pancreatectomy. J Am Coll Surg. 2020; 230:393–402.12. Shirakawa S, Matsumoto I, Toyama H, Shinzeki M, Ajiki T, Fukumoto T, et al. Pancreatic volumetric assessment as a predictor of new-onset diabetes following distal pancreatectomy. J Gastrointest Surg. 2012; 16:2212–9.13. Kang JS, Jang JY, Kang MJ, Kim E, Jung W, Chang J, et al. Endocrine function impairment after distal pancreatectomy: incidence and related factors. World J Surg. 2016; 40:440–6.14. Maxwell DW, Jajja MR, Tariq M, Mahmooth Z, Galindo RJ, Sweeney JF, et al. Development of diabetes after pancreaticoduodenectomy: results of a 10-year series using prospective endocrine evaluation. J Am Coll Surg. 2019; 228:400–12.15. Ishida J, Toyama H, Matsumoto I, Shirakawa S, Terai S, Yamashita H, et al. Glucose tolerance after pancreatectomy: a prospective observational follow-up study of pancreaticoduodenectomy and distal pancreatectomy. J Am Coll Surg. 2021; 233:753–62.16. Niwano F, Babaya N, Hiromine Y, Matsumoto I, Kamei K, Noso S, et al. Glucose metabolism after pancreatectomy: opposite extremes between pancreaticoduodenectomy and distal pancreatectomy. J Clin Endocrinol Metab. 2021; 106:e2203–14.17. Kim S, Yoon YS, Han HS, Cho JY, Choi Y, Lee B. Evaluation of a single surgeon’s learning curve of laparoscopic pancreaticoduodenectomy: risk-adjusted cumulative summation analysis. Surg Endosc. 2021; 35:2870–8.18. Lee B, Yoon YS, Kang CM, Choi M, Lee JS, Hwang HK, et al. Fistula risk score-adjusted comparison of postoperative pancreatic fistula following laparoscopic vs open pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2021; 28:1089–97.19. Mendoza AS 3rd, Han HS, Yoon YS, Cho JY, Choi Y. Laparoscopy-assisted pancreaticoduodenectomy as minimally invasive surgery for periampullary tumors: a comparison of short-term clinical outcomes of laparoscopy-assisted pancreaticoduodenectomy and open pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2015; 22:819–24.20. Kwon JH, Kim SC, Shim IK, Song KB, Lee JH, Hwang DW, et al. Factors affecting the development of diabetes mellitus after pancreatic resection. Pancreas. 2015; 44:1296–303.21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.22. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999; 22:1462–70.23. Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009; 32:335–41.24. Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med. 1995; 12:931.25. Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000; 23:295–301.26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–12.27. Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, et al. Regional differences in islet distribution in the human pancreas: preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013; 8:e67454.28. Ionescu-Tirgoviste C, Gagniuc PA, Gubceac E, Mardare L, Popescu I, Dima S, et al. A 3D map of the islet routes throughout the healthy human pancreas. Sci Rep. 2015; 5:14634.29. Mingrone G, Cummings DE. Changes of insulin sensitivity and secretion after bariatric/metabolic surgery. Surg Obes Relat Dis. 2016; 12:1199–205.30. Mezza T, Muscogiuri G, Sorice GP, Clemente G, Hu J, Pontecorvi A, et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes. 2014; 63:994–1007.31. Mori Y, Ohtsuka T, Tsutsumi K, Yasui T, Ueda J, Takahata S, et al. Different incretin responses after pancreatoduodenectomy and distal pancreatectomy. Pancreas. 2012; 41:455–60.32. Padoan A, Plebani M, Basso D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity. Int J Mol Sci. 2019; 20:676.33. Tabata H, Hirayama J, Sowa R, Furuta H, Negoro T, Sanke T, et al. Islet amyloid polypeptide (IAPP/amylin) causes insulin resistance in perfused rat hindlimb muscle. Diabetes Res Clin Pract. 1992; 15:57–61.34. Permert J, Larsson J, Westermark GT, Herrington MK, Christmanson L, Pour PM, et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N Engl J Med. 1994; 330:313–8.35. Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013; 10:423–33.36. Liu J, Knezetic JA, Strommer L, Permert J, Larsson J, Adrian TE. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J Clin Endocrinol Metab. 2000; 85:1232–8.37. Roh E, Kim KM, Park KS, Kim YJ, Chun EJ, Choi SH, et al. Comparison of pancreatic volume and fat amount linked with glucose homeostasis between healthy Caucasians and Koreans. Diabetes Obes Metab. 2018; 20:2642–52.38. Wu SC, Fu CY, Muo CH, Chang YJ. Splenectomy in trauma patients is associated with an increased risk of postoperative type II diabetes: a nationwide population-based study. Am J Surg. 2014; 208:811–6.39. Park S, Hong SM, Ahn IS. Can splenocytes enhance pancreatic beta-cell function and mass in 90% pancreatectomized rats fed a high fat diet? Life Sci. 2009; 84:358–63.40. Glowka TR, von Websky M, Pantelis D, Manekeller S, Standop J, Kalff JC, et al. Risk factors for delayed gastric emptying following distal pancreatectomy. Langenbecks Arch Surg. 2016; 401:161–7.41. Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia. 1993; 36:857–62.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of robotic surgery for pancreatic tumors
- Pancreaticoduodenectomy and Pancreatic Resection in Chronic Pancreatitis
- Surgical Management of Pancreatic Cancer
- Utilization of end to side inverted mattress pancreaticojejunostomy for Duval procedure: A case report
- Extended distal pancreatectomy for advanced pancreatic neck cancer