Endocrinol Metab.
2023 Aug;38(4):455-461. 10.3803/EnM.2023.1711.
Beyond Acute COVID-19: Investigating the Incidence of Subacute Thyroiditis in Long COVID-19 in Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Health Insurance Review and Assessment Service, Wonju, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Konkuk University Hospital, Konkuk University School of Medicine, Seoul, Korea
- KMID: 2545278
- DOI: http://doi.org/10.3803/EnM.2023.1711
Abstract
- Background
The correlation between acute coronavirus disease 2019 (COVID-19) and subacute thyroiditis (SAT) has not been clearly investigated in “long COVID” patients. We aimed to investigate the incidence of SAT during convalescence and after the acute phase of COVID-19, comparing with that of the general population.
Methods
Data from a total of 422,779 COVID-19 patients and a control group of 2,113,895 individuals were analyzed. The index date was defined as the date 3 months after confirmation of COVID-19. The incidence rate (IR) of SAT and hazard ratios (HRs) were calculated per 100,000 persons. Subgroup analysis included analysis of HRs 90–179 and 180 days post-COVID-19 diagnosis; and additional analysis was conducted according to hospitalization status, sex, and age group.
Results
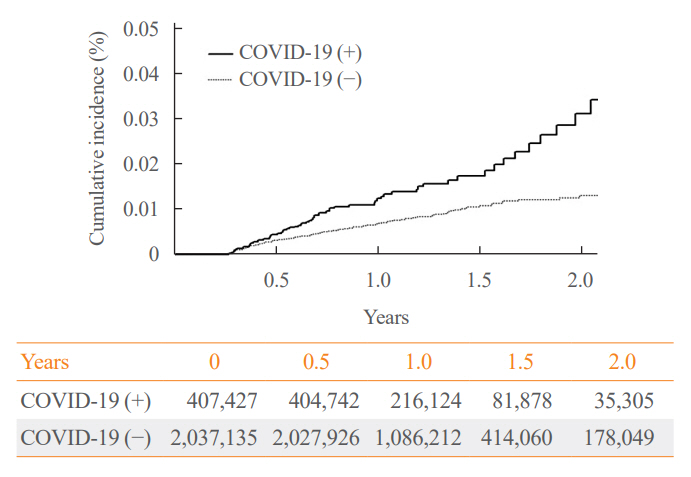
The IR of SAT was 17.28 per 100,000 persons (95% confidence interval [CI], 12.56 to 23.20) in the COVID-19 group and 8.63 (95% CI, 6.37 to 11.45) in the control group. The HR of COVID-19 patients was 1.76 (95% CI, 1.01 to 3.06; P=0.045). The HR of SAT was 1.39 (95% CI, 0.82 to 2.34; P=0.220) up to 6 months after the index date and 2.30 (95% CI, 1.60 to 3.30; P<0.001) beyond 6 months. The HR for SAT among COVID-19 patients was 2.00 (95% CI, 1.41 to 2.83) in hospitalized patients and 1.76 (95% CI, 1.01 to 3.06) in non-hospitalized patients compared to the control group. The IR of SAT was 27.09 (95% CI, 20.04 to 35.82) for females and 6.47 (95% CI, 3.34 to 11.30) for males. In the 19 to 64 age group, the IR of SAT was 18.19 (95% CI, 13.70 to 23.67), while the IR was 9.18 (95% CI, 7.72 to 10.84) in the 65 to 69 age group.
Conclusion
SAT could be a potential long-term complication of COVID-19. Long-term surveillance for thyroid dysfunction is needed especially in hospitalized, female and young-aged subjects.
Keyword
Figure
Reference
-
1. World Health Organization. COVID-19 weekly epidemiological update, edition 115, 26 October 2022 [Internet]. Geneva: WHO;2022. [cited 2023 Jul 11]. Available from: https://apps.who.int/iris/handle/10665/363853.2. Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020; 181:1016–35.3. Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential interaction between SARS-CoV-2 and thyroid: a review. Endocrinology. 2021; 162:bqab004.4. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020; 323:1843–4.5. Lazartigues E, Qadir MM, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology. 2020; 161:bqaa108.6. Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021; 5:bvab076.7. Zhang Y, Lin F, Tu W, Zhang J, Choudhry AA, Ahmed O, et al. Thyroid dysfunction may be associated with poor outcomes in patients with COVID-19. Mol Cell Endocrinol. 2021; 521:111097.8. Boguslawska J, Godlewska M, Gajda E, Piekielko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J. 2022; 11:e210024.9. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020; 183:381–7.10. Giovanella L, Ruggeri RM, Ovcaricek PP, Campenni A, Treglia G, Deandreis D. Prevalence of thyroid dysfunction in patients with COVID-19: a systematic review. Clin Transl Imaging. 2021; 9:233–40.11. Naguib R. Potential relationships between COVID-19 and the thyroid gland: an update. J Int Med Res. 2022; 50:3000605221082898.12. Ladds E, Rushforth A, Wieringa S, Taylor S, Rayner C, Husain L, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020; 20:1144.13. Yong SJ, Halim A, Halim M, Liu S, Aljeldah M, Al Shammari BR, et al. Inflammatory and vascular biomarkers in post-COVID-19 syndrome: a systematic review and meta-analysis of over 20 biomarkers. Rev Med Virol. 2023; 33:e2424.14. Centers for Disease Control and Prevention. Post-COVID conditions: CDC science [Internet]. Atlanta: CDC;2022. [cited 2023 Jul 11]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-science.html.15. Lui DT, Lee CH, Chow WS, Lee AC, Tam AR, Fong CH, et al. Insights from a prospective follow-up of thyroid function and autoimmunity among COVID-19 survivors. Endocrinol Metab (Seoul). 2021; 36:582–9.16. Korea Disease Control and Prevention Agency. Long COVID [Internet]. Cheongju: KDCA;2023. [cited 2023 Jul 11]. Available from: https://ncv.kdca.go.kr/hcp/page.do?mid=0102.17. Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020; 7:ofaa509.18. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020; 383:1757–66.19. Yin T, Li Y, Ying Y, Luo Z. Prevalence of comorbidity in Chinese patients with COVID-19: systematic review and meta-analysis of risk factors. BMC Infect Dis. 2021; 21:200.20. Sunada N, Honda H, Nakano Y, Yamamoto K, Tokumasu K, Sakurada Y, et al. Hormonal trends in patients suffering from long COVID symptoms. Endocr J. 2022; 69:1173–81.21. Boaventura P, Macedo S, Ribeiro F, Jaconiano S, Soares P. Post-COVID-19 condition: where are we now? Life (Basel). 2022; 12:517.22. Ahn HY, Choi HS, Ha S, Cho SW. Incidence of subacute thyroiditis during the COVID-19 pandemic in South Korea using the National Health Insurance Service Database. Thyroid. 2022; 32:1299–306.23. Domin R, Szczepanek-Parulska E, Dadej D, Ruchala M. Subacute thyroiditis: literature overview and COVID-19. J Med Sci. 2020; 89:e472.24. Brancatella A, Viola N, Santini F, Latrofa F. COVID-induced thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2023; 37:101742.25. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020; 8:739–41.26. Darvishi M, Nazer MR, Shahali H, Nouri M. Association of thyroid dysfunction and COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022; 13:947594.27. Lui DT, Fung MM, Chiu KW, Lee CH, Chow WS, Lee AC, et al. Higher SARS-CoV-2 viral loads correlated with smaller thyroid volumes on ultrasound among male COVID-19 survivors. Endocrine. 2021; 74:205–14.28. Stasiak M, Michalak R, Stasiak B, Lewinski A. Clinical characteristics of subacute thyroiditis is different than it used to be: current state based on 15 years own material. Neuro Endocrinol Lett. 2019; 39:489–95.29. Stasiak M, Lewinski A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021; 22:1027–39.30. Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020; 12:e8094.31. Gu J, Yin J, Zhang M, Li J, Wu Y, Chen J, et al. Study on the clinical significance of ACE2 and its age-related expression. J Inflamm Res. 2021; 14:2873–82.32. Lui DT, Lee CH, Chow WS, Lee AC, Tam AR, Fong CH, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab. 2021; 106:e926–35.33. Lui DT, Tsoi KH, Lee CH, Cheung CY, Fong CH, Lee AC, et al. A prospective follow-up on thyroid function, thyroid autoimmunity and long COVID among 250 COVID-19 survivors. Endocrine. 2023; 80:380–91.34. Monzani F, Caraccio N, Casolaro A, Lombardo F, Moscato G, Murri L, et al. Long-term interferon beta-1b therapy for MS: is routine thyroid assessment always useful? Neurology. 2000; 55:549–52.35. Kreisler A, de Seze J, Stojkovic T, Delisse B, Combelles M, Verier A, et al. Multiple sclerosis, interferon beta and clinical thyroid dysfunction. Acta Neurol Scand. 2003; 107:154–7.36. Frisullo G, Calabrese M, Tortorella C, Paolicelli D, Ragonese P, Annovazzi P, et al. Thyroid autoimmunity and dysfunction in multiple sclerosis patients during long-term treatment with interferon beta or glatiramer acetate: an Italian multicenter study. Mult Scler. 2014; 20:1265–8.37. Watad A, David P, Brown S, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants and thyroid autoimmunity. Front Endocrinol (Lausanne). 2017; 7:150.