J Korean Med Sci.
2023 Aug;38(33):e258. 10.3346/jkms.2023.38.e258.
Epigenetic Regulation of the Expression of T Cell Stimulatory and Inhibitory Factors by Histone H3 Lysine Modification Enzymes and Its Prognostic Roles in Glioblastoma
- Affiliations
-
- 1Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2Department of New Biology, Daegu Gyeongbuk Institute of Science and Technology, Daegu, Korea
- 3Well Aging Research Center, Division of Biotechnology, Daegu Gyeongbuk Institute of Science and Technology, Daegu, Korea
- 4Translational Responsive Medicine Center, Daegu Gyeongbuk Institute of Science and Technology, Daegu, Korea
- 5Department of Pathology, School of Dentistry, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- 6Department of Anesthesiology and Pain Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 7Cancer Research Institute, Clinomics Inc., Suwon, Korea
- KMID: 2545210
- DOI: http://doi.org/10.3346/jkms.2023.38.e258
Abstract
- Background
This study aimed to identify the specific T cell co-stimulatory and co-inhibitory factors that play prognostic roles in patients with glioblastoma. Additionally, the unique histone H3 modification enzymes that regulate the expression levels of these specific costimulatory and co-inhibitory factors were investigated.
Methods
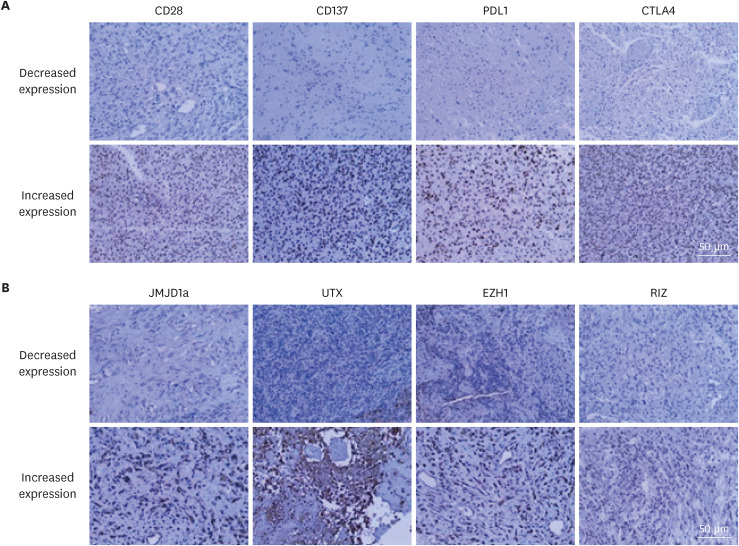
The medical records of 84 patients newly diagnosed with glioblastoma at our institution from January 2006 to December 2020 were retrospectively reviewed. Immunohistochemical (IHC) staining for T cell co-stimulatory factors (CD27, CD28, CD137, OX40, and ICOS), T cell co-inhibitory factors (CTLA4, PD1, PD-L1, TIM3, and CD200R), and histone H3 lysine modification enzymes (MLL4, RIZ, EZH1, NSD2, KDM5c, JMJD1a, UTX, and JMJD5) was performed on archived paraffin-embedded tissues obtained by biopsy or resection. Quantitative real time-polymerase chain reaction (qRT-PCR) was performed for specific factors, which demonstrated causal relationships, in order to validate the findings of the IHC examinations.
Results
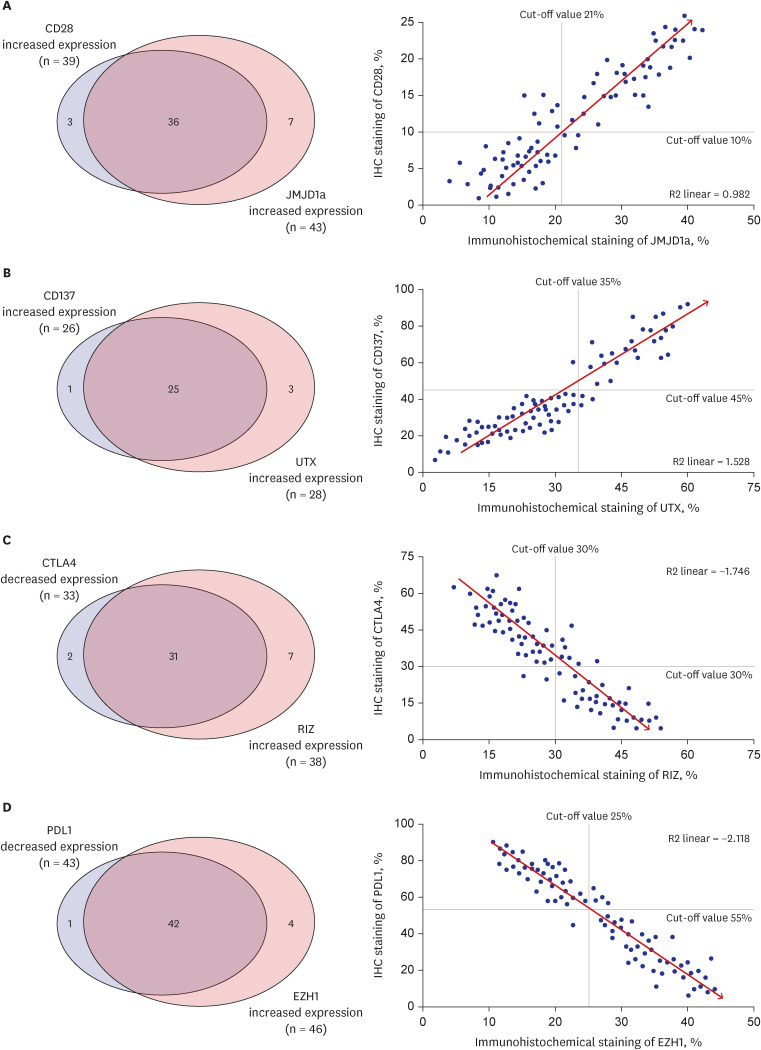
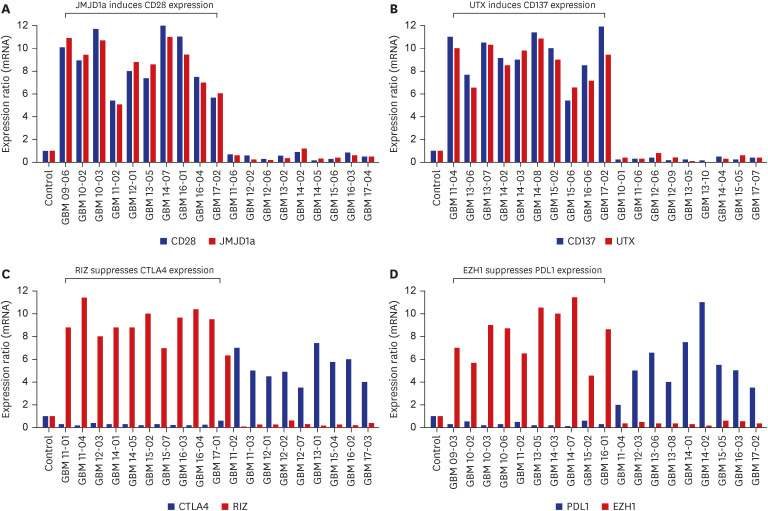
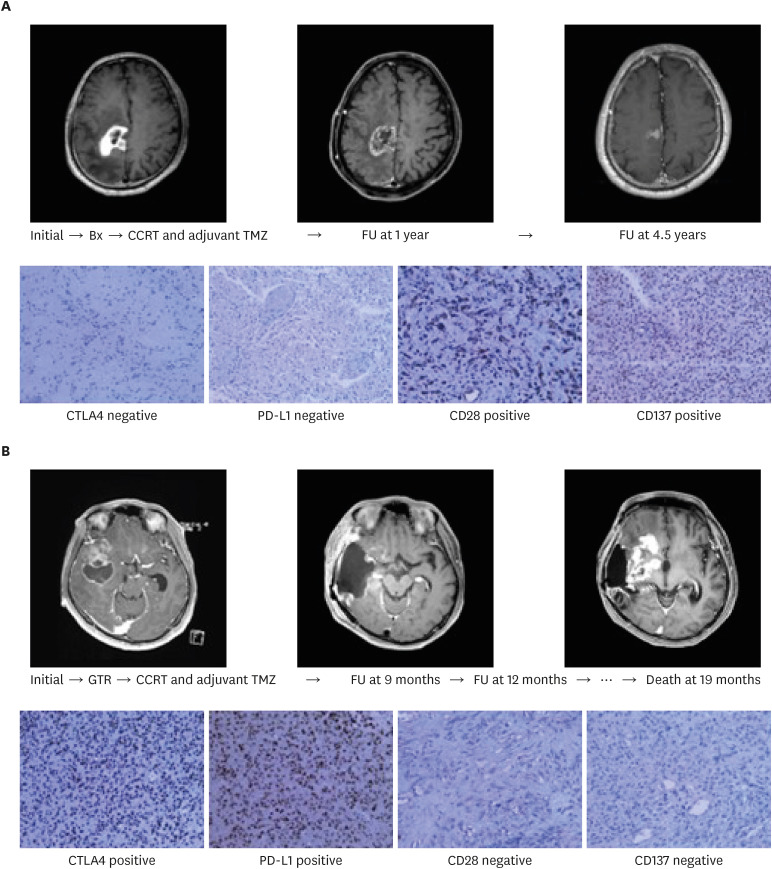
The mean follow-up duration was 27.5 months (range, 4.1–43.5 months). During this period, 76 patients (90.5%) died, and the mean OS was 19.4 months (95% confidence interval, 16.3–20.9 months). Linear positive correlations were observed between the expression levels of CD28 and JMJD1a (R2 linear = 0.982) and those of CD137 and UTX (R2 linear = 1.528). Alternatively, significant negative correlations were observed between the expression levels of CTLA4 and RIZ (R2 linear = −1.746) and those of PD-L1 and EZH1 (R2 linear = −2.118); relationships were confirmed by qRT-PCR. In the multivariate analysis, increased expression levels of CD28 (P = 0.042), and CD137 (P = 0.009), and decreased expression levels of CTLA4 (P = 0.003), PD-L1 (P = 0.020), and EZH1 (P = 0.040) were significantly associated with longer survival.
Conclusion
These findings suggest that the expression of certain T cell co-stimulatory factors, such as CD28 and CD 137, and co-inhibitory factors, such as CTLA4 and PD-L1 are associated with prognosis of glioblastoma patients.
Figure
Reference
-
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2011–2015. Neuro-Oncology. 2018; 20(suppl_4):iv1–i86. PMID: 30445539.2. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. 2021; 23(8):1231–1251. PMID: 34185076.3. Dho YS, Jung KW, Ha J, Seo Y, Park CK, Won YJ, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5(1):16–23. PMID: 28516074.4. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005; 352(10):997–1003. PMID: 15758010.5. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10(5):459–466. PMID: 19269895.6. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015; 523(7560):337–341. PMID: 26030524.7. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017; 377(14):1345–1356. PMID: 28889792.8. Buerki RA, Chheda ZS, Okada H. Immunotherapy of primary brain tumors: facts and hopes. Clin Cancer Res. 2018; 24(21):5198–5205. PMID: 29871908.9. Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, et al. OS10.3 randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-Oncology. 2017; 19(Suppl 3):iii21.10. Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncology. 2018; 20(5):674–686. PMID: 29106665.11. Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, et al. Randomized phase II and biomarker study of pembrolizumab plus bevacizumab versus pembrolizumab alone for patients with recurrent glioblastoma. Clin Cancer Res. 2021; 27(4):1048–1057. PMID: 33199490.12. Sampson JH, Omuro AM, Preusser M, Lim M, Butowski NA, Cloughesy TF, et al. A randomized, phase 3, open-label study of nivolumab versus temozolomide (TMZ) in combination with radiotherapy (RT) in adult patients (pts) with newly diagnosed, O-6-methylguanine DNA methyl-transferase (MGMT)-unmethylated glioblastoma (GBM): CheckMate-498. J Clin Oncol. 2016; 34(15):TPS2079.13. Weller M, Vlahovic G, Khasraw M, Brandes AA, Zwirtes R, Tatsuoka K, et al. A randomized phase 2, single-blind study of temozolomide (TMZ) and radiotherapy (RT) combined with nivolumab or placebo (PBO) in newly diagnosed adult patients (pts) with tumor O6-methylguanine DNA methyltransferase (MGMT)-methylated glioblastoma (GBM)—CheckMate-548. Ann Oncol. 2016; 27(Suppl 6):VI113.14. Han S, Ma E, Wang X, Yu C, Dong T, Zhan W, et al. Rescuing defective tumor-infiltrating T-cell proliferation in glioblastoma patients. Oncol Lett. 2016; 12(4):2924–2929. PMID: 27703529.15. Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018; 24(16):3792–3802. PMID: 29593027.16. Yeo AT, Charest A. Immune checkpoint blockade biology in mouse models of glioblastoma. J Cell Biochem. 2017; 118(9):2516–2527. PMID: 28230277.17. Özdemir BC, Siefker-Radtke AO, Campbell MT, Subudhi SK. Current and future applications of novel immuno-therapies in urological oncology: a critical review of the literature. Eur Urol Focus. 2018; 4(3):442–454. PMID: 29056275.18. Casado JG, Soto R, DelaRosa O, Peralbo E, del Carmen Muñoz-Villanueva M, Rioja L, et al. CD8 T cells expressing NK associated receptors are increased in melanoma patients and display an effector phenotype. Cancer Immunol Immunother. 2005; 54(12):1162–1171. PMID: 15889254.19. Fourcade J, Sun Z, Chauvin JM, Ka M, Davar D, Pagliano O, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018; 3(14):e121157. PMID: 30046006.20. Kim HD, Park S, Jeong S, Lee YJ, Lee H, Kim CG, et al. 4-1BB delineates distinct activation status of exhausted tumor-infiltrating CD8+ T cells in hepatocellular carcinoma. Hepatology. 2020; 71(3):955–971. PMID: 31353502.21. Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res. 2014; 20(1):44–55. PMID: 24045181.22. Ladányi A, Somlai B, Gilde K, Fejös Z, Gaudi I, Tímár J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004; 10(2):521–530. PMID: 14760073.23. Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134). Am J Surg. 2002; 183(5):512–518. PMID: 12034383.24. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017; 114(19):4993–4998. PMID: 28446615.25. Zhang Y, Luo Y, Qin SL, Mu YF, Qi Y, Yu MH, et al. The clinical impact of ICOS signal in colorectal cancer patients. Oncoimmunology. 2016; 5(5):e1141857. PMID: 27467961.26. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017; 35(21):2439–2449. PMID: 28640707.27. Lee YM, Kim SH, Kim MS, Kim DC, Lee EH, Lee JS, et al. Epigenetic role of histone lysine methyltransferase and demethylase on the expression of transcription factors associated with the epithelial-to-mesenchymal transition of lung adenocarcinoma metastasis to the brain. Cancers (Basel). 2020; 12(12):3632–3653. PMID: 33291558.28. Eng J. Receiver operating characteristic analysis: a primer. Acad Radiol. 2005; 12(7):909–916. PMID: 16039544.29. Wiencke JK, Accomando WP, Zheng S, Patoka J, Dou X, Phillips JJ, et al. Epigenetic biomarkers of T-cells in human glioma. Epigenetics. 2012; 7(12):1391–1402. PMID: 23108258.30. Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, et al. DNA methylation controls Foxp3 gene expression. Eur J Immunol. 2008; 38(6):1654–1663. PMID: 18493985.31. Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007; 5(2):e38. PMID: 17298177.32. Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005; 17(2):105–119. PMID: 15737572.33. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015; 161(2):205–214. PMID: 25860605.34. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12(4):252–264. PMID: 22437870.35. Binder DC, Davis AA, Wainwright DA. Immunotherapy for cancer in the central nervous system: Current and future directions. Oncoimmunology. 2015; 5(2):e1082027. PMID: 27057463.36. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013; 86(2):343–349. PMID: 23462419.37. Reardon DA, Freeman G, Wu C, Chiocca EA, Wucherpfennig KW, Wen PY, et al. Immunotherapy advances for glioblastoma. Neuro-Oncology. 2014; 16(11):1441–1458. PMID: 25190673.38. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363(8):711–723. PMID: 20525992.39. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366(26):2443–2454. PMID: 22658127.40. Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007; 13(7):2158–2167. PMID: 17404100.41. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018; 359(6382):1350–1355. PMID: 29567705.42. Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017; 214(4):895–904. PMID: 28302645.43. Diskin B, Adam S, Cassini MF, Sanchez G, Liria M, Aykut B, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020; 21(4):442–454. PMID: 32152508.44. Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022; 19(5):287–305. PMID: 35132224.45. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010; 116(17):3268–3277. PMID: 20628145.46. Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016; 34(23):2690–2697. PMID: 27069084.47. George J, Saito M, Tsuta K, Iwakawa R, Shiraishi K, Scheel AH, et al. Genomic amplification of CD274 (PD-L1) in small-cell lung cancer. Clin Cancer Res. 2017; 23(5):1220–1226. PMID: 27620277.48. Lu C, Paschall AV, Shi H, Savage N, Waller JL, Sabbatini ME, et al. The MLL1-H3K4me3 axis-mediated PD-L1 expression and pancreatic cancer immune evasion. J Natl Cancer Inst. 2017; 109(6):djw283. PMID: 28131992.49. Xiao G, Jin LL, Liu CQ, Wang YC, Meng YM, Zhou ZG, et al. EZH2 negatively regulates PD-L1 expression in hepatocellular carcinoma. J Immunother Cancer. 2019; 7(1):300. PMID: 31727135.50. Emran AA, Chatterjee A, Rodger EJ, Tiffen JC, Gallagher SJ, Eccles MR, et al. Targeting DNA methylation and EZH2 activity to overcome melanoma resistance to immunotherapy. Trends Immunol. 2019; 40(4):328–344. PMID: 30853334.51. Zhang Y, Xiang C, Wang Y, Duan Y, Liu C, Zhang Y. PD-L1 promoter methylation mediates the resistance response to anti-PD-1 therapy in NSCLC patients with EGFR-TKI resistance. Oncotarget. 2017; 8(60):101535–101544. PMID: 29254184.52. Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014; 28(6):1280–1288. PMID: 24270737.53. Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, et al. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010; 17(11):1381–1385. PMID: 20727764.54. McVicar DW, Davis DF, Merchant RE. In vitro analysis of the proliferative potential of T cells from patients with brain tumor: glioma-associated immunosuppression unrelated to intrinsic cellular defect. J Neurosurg. 1992; 76(2):251–260. PMID: 1730954.55. El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology. 2006; 8(3):234–243. PMID: 16723631.56. Bakhshi TJ, Georgel PT. Genetic and epigenetic determinants of diffuse large B-cell lymphoma. Blood Cancer J. 2020; 10(12):123. PMID: 33277464.57. Béguelin W, Rivas MA, Calvo Fernández MT, Teater M, Purwada A, Redmond D, et al. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat Commun. 2017; 8(1):877. PMID: 29026085.58. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018; 378(15):1396–1407. PMID: 29641966.59. Wagener N, Macher-Goeppinger S, Pritsch M, Hüsing J, Hoppe-Seyler K, Schirmacher P, et al. Enhancer of zeste homolog 2 (EZH2) expression is an independent prognostic factor in renal cell carcinoma. BMC Cancer. 2010; 10(1):524. PMID: 20920340.60. Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012; 106(2):243–247. PMID: 22187039.61. Orzan F, Pellegatta S, Poliani PL, Pisati F, Caldera V, Menghi F, et al. Enhancer of Zeste 2 (EZH2) is up-regulated in malignant gliomas and in glioma stem-like cells. Neuropathol Appl Neurobiol. 2011; 37(4):381–394. PMID: 20946108.62. Yin Y, Qiu S, Peng Y. Functional roles of enhancer of zeste homolog 2 in gliomas. Gene. 2016; 576(1 Pt 2):189–194. PMID: 26435191.63. Bian EB, Li J, He XJ, Zong G, Jiang T, Li J, et al. Epigenetic modification in gliomas: role of the histone methyltransferase EZH2. Expert Opin Ther Targets. 2014; 18(10):1197–1206. PMID: 25046371.64. Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell. 2014; 54(5):716–727. PMID: 24905005.65. Hyun K, Jeon J, Park K, Kim J. Writing, erasing and reading histone lysine methylations. Exp Mol Med. 2017; 49(4):e324. PMID: 28450737.66. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018; 13(1):e0190783. PMID: 29351281.67. Hara T, Chanoch-Myers R, Mathewson ND, Myskiw C, Atta L, Bussema L, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021; 39(6):779–792.e11. PMID: 34087162.68. Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016; 22(8):1856–1864. PMID: 27084739.