Ann Surg Treat Res.
2023 Aug;105(2):82-90. 10.4174/astr.2023.105.2.82.
Efficacy of ferric carboxymaltose in iron deficiency anemia patients scheduled for pancreaticoduodenectomy
- Affiliations
-
- 1Department of General Surgery, Osan Hankook Hospital, Osan, Korea
- 2Center for Liver and Pancreatobiliary Cancer, National Cancer Center, Goyang, Korea
- 3Biomedical Statistics Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, Korea
- 4Center for Gastric Cancer, National Cancer Center, Goyang, Korea
- 5Department of Surgery, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- KMID: 2545159
- DOI: http://doi.org/10.4174/astr.2023.105.2.82
Abstract
- Purpose
Perioperative transfusion is reported to be an independent risk factor not only for postoperative complications but also for early recurrence of periampullary carcinoma after pancreaticoduodenectomy (PD). The purpose of this study was to evaluate the safety and efficacy of ferric carboxymaltose (FCM) in reducing the need for perioperative transfusion in iron deficiency anemia patients scheduled for PD.
Methods
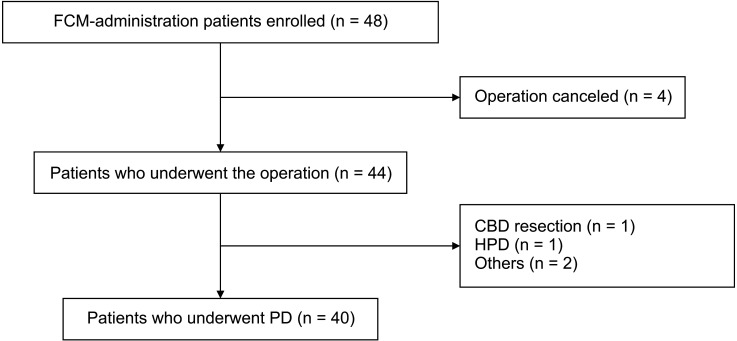
Twenty-two male patients (hemoglobin [Hb] 7 to <13 g/dL) and 18 female patients (Hb 7 to <12 g/dL) were enrolled in the study group and administered FCM 1–3 weeks before PD. The perioperative transfusion rate was the primary endpoint; morbidity, length of postoperative hospital stay, change in hematological parameters after FCM injection, and adverse effects of FCM were also investigated.
Results
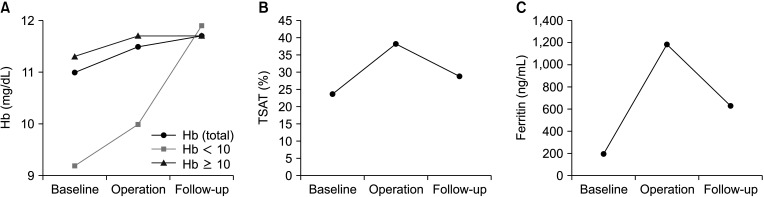
The perioperative transfusion rate of the study group was 22.5% (9 of 40). Hb level was significantly higher on the day of the operation compared to baseline (P < 0.001). Levels of Hb, transferrin saturation, and ferritin were higher at the follow-up compared to baseline (P = 0.008, P = 0.033, and P < 0.001, respectively).
Conclusions
FCM administration was associated with a reduced need for perioperative transfusion and can safely stabilize hematological parameters.
Keyword
Figure
Reference
-
1. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007; 142:20–25. PMID: 17629996.
Article2. Wellner UF, Kulemann B, Lapshyn H, Hoeppner J, Sick O, Makowiec F, et al. Postpancreatectomy hemorrhage: incidence, treatment, and risk factors in over 1,000 pancreatic resections. J Gastrointest Surg. 2014; 18:464–475. PMID: 24448997.
Article3. Asari S, Matsumoto I, Toyama H, Yamaguchi M, Okada T, Shinzeki M, et al. Recommendation of treatment strategy for postpancreatectomy hemorrhage: Lessons from a single-center experience in 35 patients. Pancreatology. 2016; 16:454–463. PMID: 26935829.
Article4. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4:567–579. PMID: 11307091.
Article5. Park SJ, Kim SW, Jang JY, Lee KU, Park YH. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002; 26:487–492. PMID: 11910485.
Article6. Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg. 2007; 94:466–472. PMID: 17330243.
Article7. Hallet J, Mahar AL, Tsang ME, Lin Y, Callum J, Coburn NG, et al. The impact of peri-operative blood transfusions on post-pancreatectomy short-term outcomes: an analysis from the American College of Surgeons National Surgical Quality Improvement Program. HPB (Oxford). 2015; 17:975–982. PMID: 26301741.
Article8. Elwood NR, Martin AN, Turrentine FE, Jones RS, Zaydfudim VM. The negative effect of perioperative red blood cell transfusion on morbidity and mortality after major abdominal operations. Am J Surg. 2018; 216:487–491. PMID: 29475550.
Article9. Burrows L, Tartter P. Effect of blood transfusions on colonic malignancy recurrent rate. Lancet. 1982; 2:662.10. Kneuertz PJ, Patel SH, Chu CK, Maithel SK, Sarmiento JM, Delman KA, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011; 18:1327–1334. PMID: 21369744.
Article11. Park HM, Park SJ, Shim JR, Lee EC, Lee SD, Han SS, et al. Perioperative transfusion in pancreatoduodenectomy: the double-edged sword of pancreatic surgeons. Medicine (Baltimore). 2017; 96:e9019. PMID: 29245285.12. Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984; 64:308–310. PMID: 6234037.
Article13. Waymack JP, Gallon L, Barcelli U, Trocki O, Alexander JW. Effect of blood transfusions on immune function. III. Alterations in macrophage arachidonic acid metabolism. Arch Surg. 1987; 122:56–60. PMID: 3492188.14. Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999; 93:1770–1777. PMID: 10029607.
Article15. Innerhofer P, Tilz G, Fuchs D, Luz G, Hobisch-Hagen P, Schobersberger W, et al. Immunologic changes after transfusion of autologous or allogeneic buffy coat-poor versus WBC-reduced blood transfusions in patients undergoing arthroplasty. II. Activation of T cells, macrophages, and cell-mediated lympholysis. Transfusion. 2000; 40:821–827. PMID: 10924610.
Article16. Yoon HM, Kim YW, Nam BH, Reim D, Eom BW, Park JY, et al. Intravenous iron supplementation may be superior to observation in acute isovolemic anemia after gastrectomy for cancer. World J Gastroenterol. 2014; 20:1852–1857. PMID: 24587663.17. Khalafallah AA, Yan C, Al-Badri R, Robinson E, Kirkby BE, Ingram E, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016; 3:e415–e425. PMID: 27570088.
Article18. Kim YW, Bae JM, Park YK, Yang HK, Yu W, Yook JH, et al. Effect of intravenous ferric carboxymaltose on hemoglobin response among patients with acute isovolemic anemia following gastrectomy: the FAIRY randomized clinical trial. JAMA. 2017; 317:2097–2104. PMID: 28535237.
Article19. Martínez-Escribano C, Arteaga Moreno F, Cuesta Peredo D, Blanco Gonzalez FJ, De la Cámara-de Las Heras JM, Tarazona Santabalbina FJ. Before-and-after study of the first four years of the enhanced recovery after surgery (ERAS®) programme in older adults undergoing elective colorectal cancer surgery. Int J Environ Res Public Health. 2022; 19:15299. PMID: 36430017.
Article20. Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015; 75:101–127. PMID: 25428711.
Article21. Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. Anaemia Working Group España. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011; 107:477–478. PMID: 21841061.
Article22. Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017; 104:214–221. PMID: 28092401.23. Reim D, Kim YW, Nam BH, Kim MJ, Yook JH, Park YK, et al. FAIRY: a randomized controlled patient-blind phase III study to compare the efficacy and safety of intravenous ferric carboxymaltose (Ferinject®) to placebo in patients with acute isovolemic anemia after gastrectomy. Study protocol for a randomized controlled trial. Trials. 2014; 15:111. PMID: 24708660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacotherapeutics for iron deficiency anemia in adults
- Intravenous Iron Sucrose for Three Children with Iron Deficiency Anemia Failing to Respond to Oral Iron Therapy
- Anaphylaxis to Intravenous Ferric Carboxymaltose
- Iron deficiency anemia in childhood
- Postoperative Intravenous Ferric Carboxymaltose Reduces Transfusion Amounts after Orthopedic Hip Surgery