J Korean Med Sci.
2023 Jul;38(29):e220. 10.3346/jkms.2023.38.e220.
Machine Learning-Based Proteomics Reveals Ferroptosis in COPD PatientDerived Airway Epithelial Cells Upon Smoking Exposure
- Affiliations
-
- 1Division of Pulmonary, and Critical Care Medicine, Department of Medicine, Stanford University, Stanford, CA, USA
- 2Department of Computer Science and Engineering, Seoul National University, Seoul, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 4Interdisciplinary Program in Bioinformatics, Seoul National University, Seoul, Korea
- 5Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA, USA
- 6Graduate School of Public Health, Seoul National University, Seoul, Korea
- 7Institute of Health and Environment, Seoul National University, Seoul, Korea
- 8Transdisciplinary Department of Medicine & Advanced Technology, Seoul National University Hospital, Seoul, Korea
- 9Proteomics Core Facility, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- KMID: 2544943
- DOI: http://doi.org/10.3346/jkms.2023.38.e220
Abstract
- Background
Proteomics and genomics studies have contributed to understanding the pathogenesis of chronic obstructive pulmonary disease (COPD), but previous studies have limitations. Here, using a machine learning (ML) algorithm, we attempted to identify pathways in cultured bronchial epithelial cells of COPD patients that were significantly affected when the cells were exposed to a cigarette smoke extract (CSE).
Methods
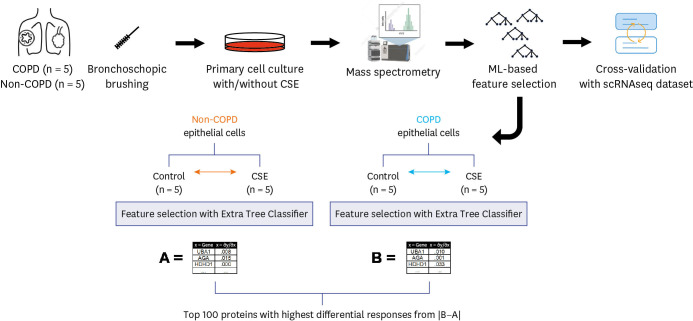
Small airway epithelial cells were collected from patients with COPD and those without COPD who underwent bronchoscopy. After expansion through primary cell culture, the cells were treated with or without CSEs, and the proteomics of the cells were analyzed by mass spectrometry. ML-based feature selection was used to determine the most distinctive patterns in the proteomes of COPD and non-COPD cells after exposure to smoke extract. Publicly available single-cell RNA sequencing data from patients with COPD (GSE136831) were used to analyze and validate our findings.
Results
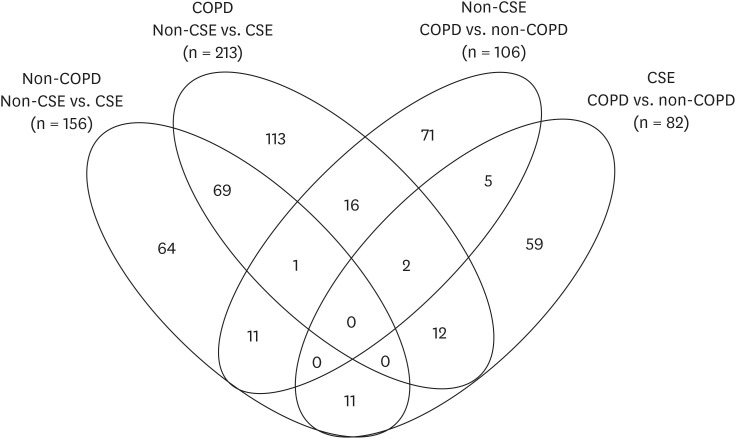
Five patients with COPD and five without COPD were enrolled, and 7,953 proteins were detected. Ferroptosis was enriched in both COPD and non-COPD epithelial cells after their exposure to smoke extract. However, the ML-based analysis identified ferroptosis as the most dramatically different response between COPD and non-COPD epithelial cells, adjusted P value = 4.172 × 10−6 , showing that epithelial cells from COPD patients are particularly vulnerable to the effects of smoke. Single-cell RNA sequencing data showed that in cells from COPD patients, ferroptosis is enriched in basal, goblet, and club cells in COPD but not in other cell types.
Conclusion
Our ML-based feature selection from proteomic data reveals ferroptosis to be the most distinctive feature of cultured COPD epithelial cells compared to non-COPD epithelial cells upon exposure to smoke extract.
Keyword
Figure
Reference
-
1. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022; 10(5):447–458. PMID: 35279265.2. Rhee CK. High prevalence of chronic obstructive pulmonary disease in Korea. Korean J Intern Med. 2016; 31(4):651–652. PMID: 27378127.3. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020; 396(10258):1204–1222. PMID: 33069326.4. Park SC, Kim DW, Park EC, Shin CS, Rhee CK, Kang YA, et al. Mortality of patients with chronic obstructive pulmonary disease: a nationwide populationbased cohort study. Korean J Intern Med. 2019; 34(6):1272–1278. PMID: 31610634.5. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of chronic obstructive pulmonary disease: 2022 report. Updated 2022. Accessed September 29, 2022. https://goldcopd.org/. .6. Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008; 12(7):703–708. PMID: 18544191.7. Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019; 381(13):1248–1256. PMID: 31553836.8. Liu Y, Liu H, Li C, Ma C, Ge W. Proteome profiling of lung tissues in chronic obstructive pulmonary disease (COPD): platelet and macrophage dysfunction contribute to the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis. 2020; 15:973–980. PMID: 32440109.9. Zhu Y, Zhou A, Li Q. Whole transcriptome analyis of human lung tissue to identify COPD-associated genes. Genomics. 2020; 112(5):3135–3141. PMID: 32470642.10. Morrow JD, Chase RP, Parker MM, Glass K, Seo M, Divo M, et al. RNA-sequencing across three matched tissues reveals shared and tissue-specific gene expression and pathway signatures of COPD. Respir Res. 2019; 20(1):65. PMID: 30940135.11. Han D, Jin J, Woo J, Min H, Kim Y. Proteomic analysis of mouse astrocytes and their secretome by a combination of FASP and StageTip-based, high pH, reversed-phase fractionation. Proteomics. 2014; 14(13-14):1604–1609. PMID: 24753479.12. Kim JE, Han D, Jeong JS, Moon JJ, Moon HK, Lee S, et al. Multisample mass spectrometry-based approach for discovering injury markers in chronic kidney disease. Mol Cell Proteomics. 2021; 20:100037. PMID: 33453410.13. Park J, Kim H, Kim SY, Kim Y, Lee JS, Dan K, et al. In-depth blood proteome profiling analysis revealed distinct functional characteristics of plasma proteins between severe and non-severe COVID-19 patients. Sci Rep. 2020; 10(1):22418. PMID: 33376242.14. Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016; 11(12):2301–2319. PMID: 27809316.15. Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011; 10(4):1794–1805. PMID: 21254760.16. Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014; 13(9):2513–2526. PMID: 24942700.17. Xie Z, Bailey A, Kuleshov MV, Clarke DJ, Evangelista JE, Jenkins SL, et al. Gene set knowledge discovery with Enrichr. Curr Protoc. 2021; 1(3):e90. PMID: 33780170.18. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020; 6(28):eaba1983. PMID: 32832599.19. Croasdell Lucchini A, Gachanja NN, Rossi AG, Dorward DA, Lucas CD. Epithelial cells and inflammation in pulmonary wound repair. Cells. 2021; 10(2):339. PMID: 33562816.20. Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res. 2006; 7(1):53. PMID: 16571143.21. Pierotti CL, Silke J. Necroptosis in chronic obstructive pulmonary disease, a smoking gun? Immunol Cell Biol. 2022; 100(2):79–82. PMID: 35023199.22. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012; 149(5):1060–1072. PMID: 22632970.23. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021; 22(4):266–282. PMID: 33495651.24. Yoshida M, Minagawa S, Araya J, Sakamoto T, Hara H, Tsubouchi K, et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 2019; 10(1):3145. PMID: 31316058.25. Mostafaei S, Kazemnejad A, Azimzadeh Jamalkandi S, Amirhashchi S, Donnelly SC, Armstrong ME, et al. Identification of novel genes in human airway epithelial cells associated with chronic obstructive pulmonary disease (COPD) using machine-based learning algorithms. Sci Rep. 2018; 8(1):15775. PMID: 30361509.26. Zhang YH, Hoopmann MR, Castaldi PJ, Simonsen KA, Midha MK, Cho MH, et al. Lung proteomic biomarkers associated with chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2021; 321(6):L1119–L1130. PMID: 34668408.27. Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020; 587(7835):619–625. PMID: 33208946.28. Yaghi A, Dolovich MB. Airway epithelial cell cilia and obstructive lung disease. Cells. 2016; 5(4):40. PMID: 27845721.29. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021; 31(2):107–125. PMID: 33268902.30. Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020; 11(2):88. PMID: 32015325.31. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020; 152:175–185. PMID: 32165281.32. Engelen MP, Orozco-Levi M, Deutz NE, Barreiro E, Hernández N, Wouters EF, et al. Glutathione and glutamate levels in the diaphragm of patients with chronic obstructive pulmonary disease. Eur Respir J. 2004; 23(4):545–551. PMID: 15083752.33. Li JY, Yao YM, Tian YP. Ferroptosis: a trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol. 2021; 12:701163. PMID: 34489948.34. DeMeo DL. Sex and gender omic biomarkers in men and women with COPD: considerations for precision medicine. Chest. 2021; 160(1):104–113. PMID: 33745988.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology of Chronic Obstructive Pulmonary Disease (COPD)
- Analysis of Airway Epithelial Cell Autoantigens Recognized by IgG Autoantibodies from Patients with Severe Asthma and Chronic Obstructive Pulmonary Disease
- Complications of Chronic Obstructive Pulmonary Disease (COPD)
- Risk Factors of Chronic Obstructive Pulmonary Disease (COPD)
- Phenotype of Chronic Obstructive Pulmonary Disease Based on Computed Tomography–Defined Underlying Pathology