J Pathol Transl Med.
2023 Jul;57(4):196-207. 10.4132/jptm.2023.06.12.
A stepwise approach to fine needle aspiration cytology of lymph nodes
- Affiliations
-
- 1Department of Hospital Pathology, The Catholic University of Korea, College of Medicine, Seoul, Korea
- 2Department of Pathology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 3Department of Pathology, Inje University Sanggye Paik Hospital, Seoul, Korea
- 4Department of Pathology, Samkwang Medical Laboratories, Seoul, Korea
- 5Department of Pathology, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- KMID: 2544327
- DOI: http://doi.org/10.4132/jptm.2023.06.12
Abstract
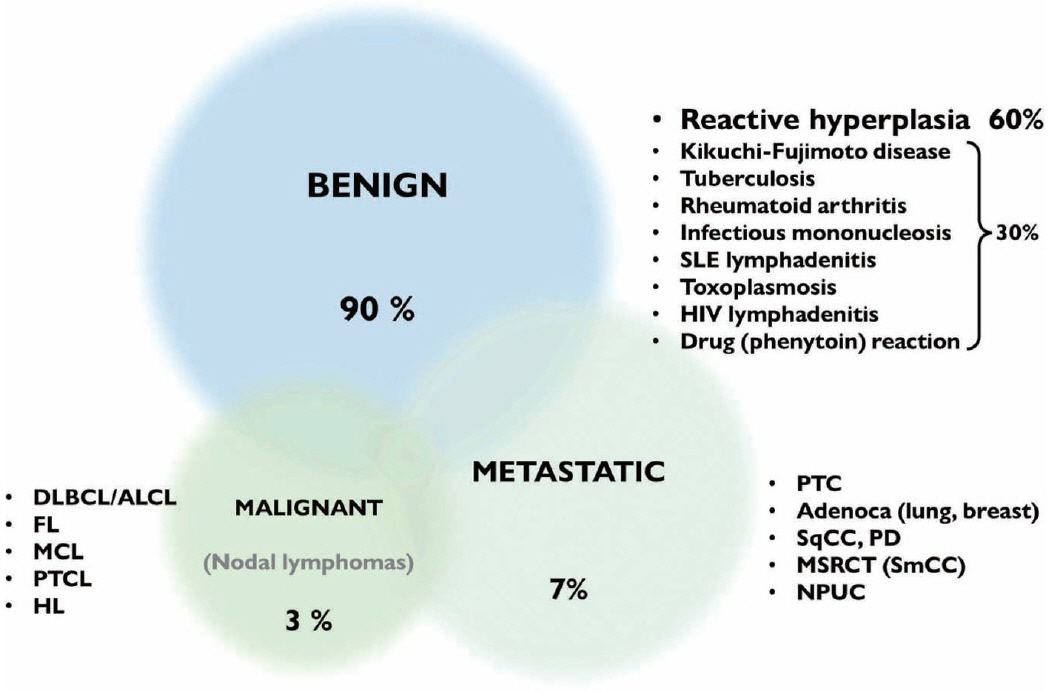
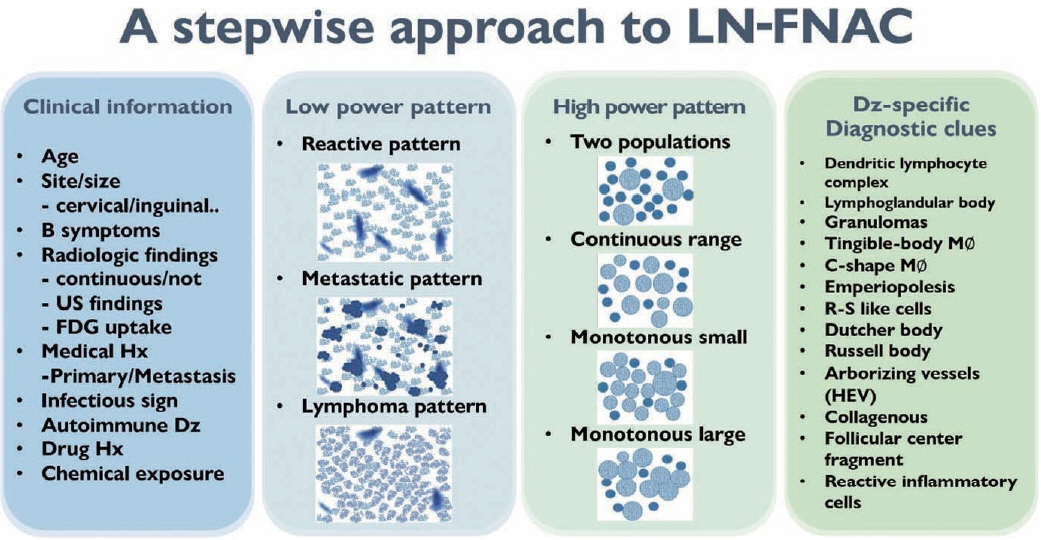
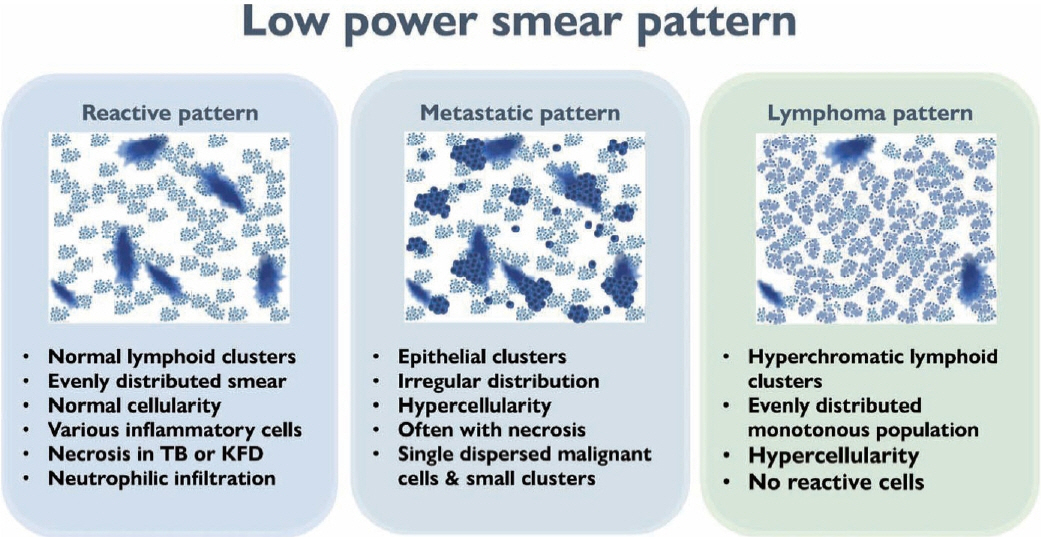
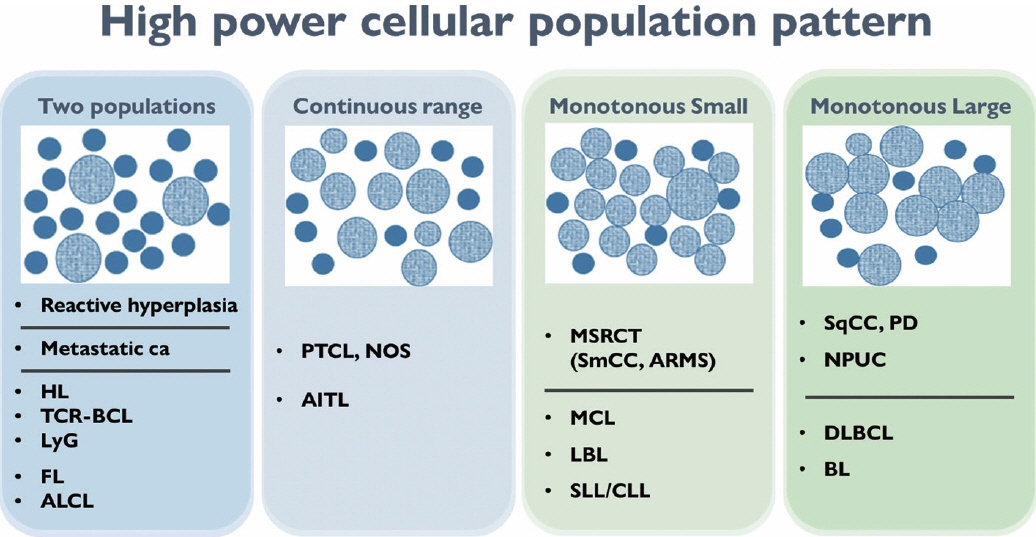
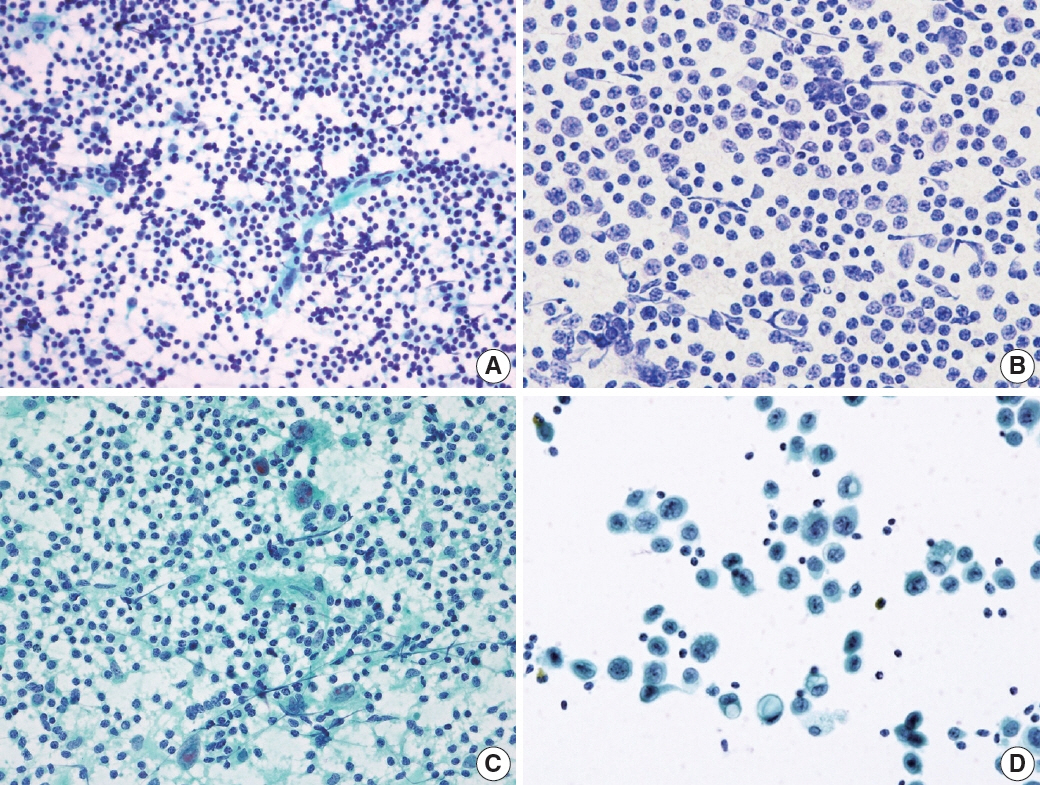
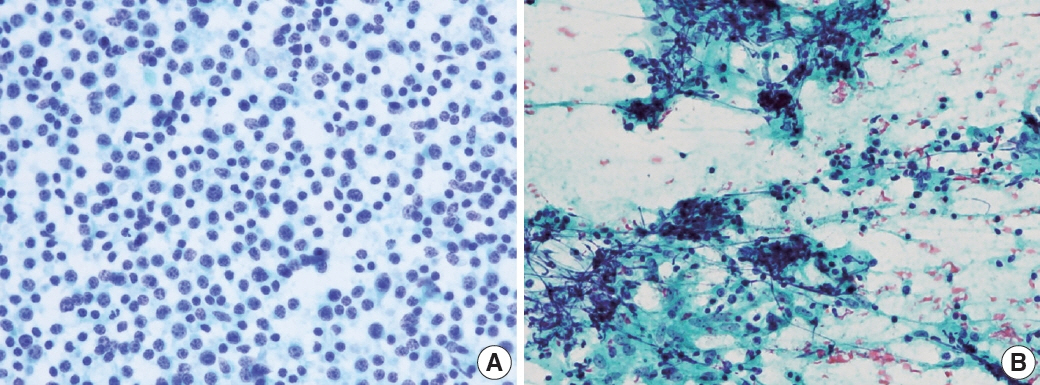
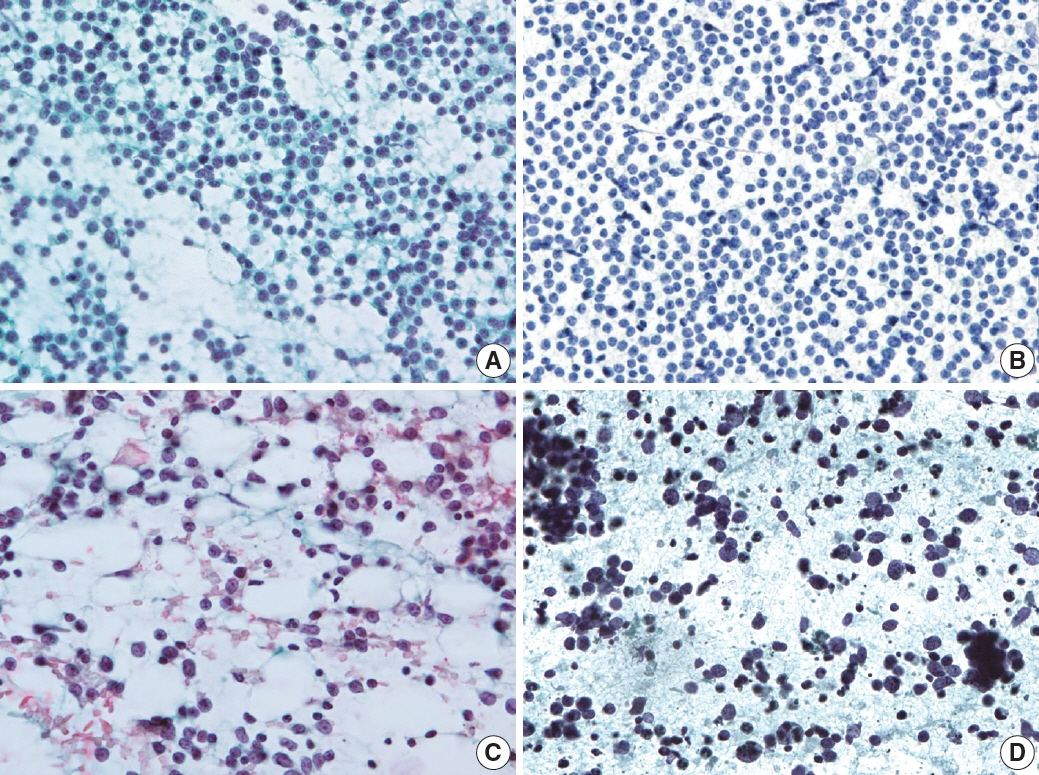
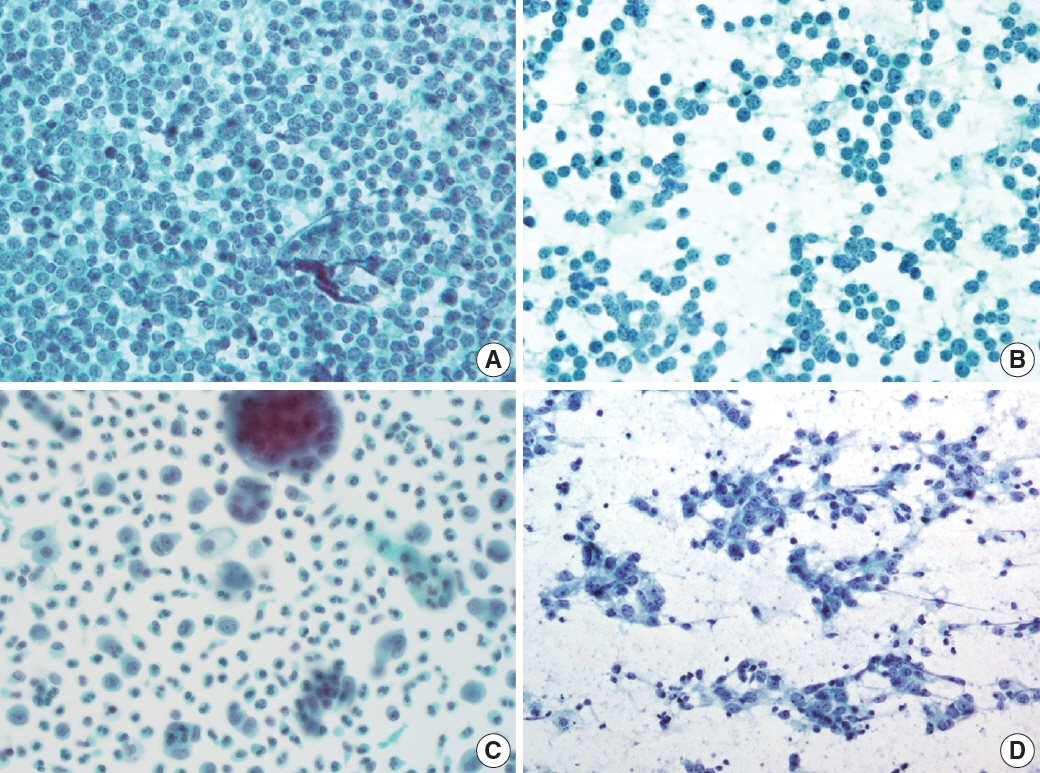
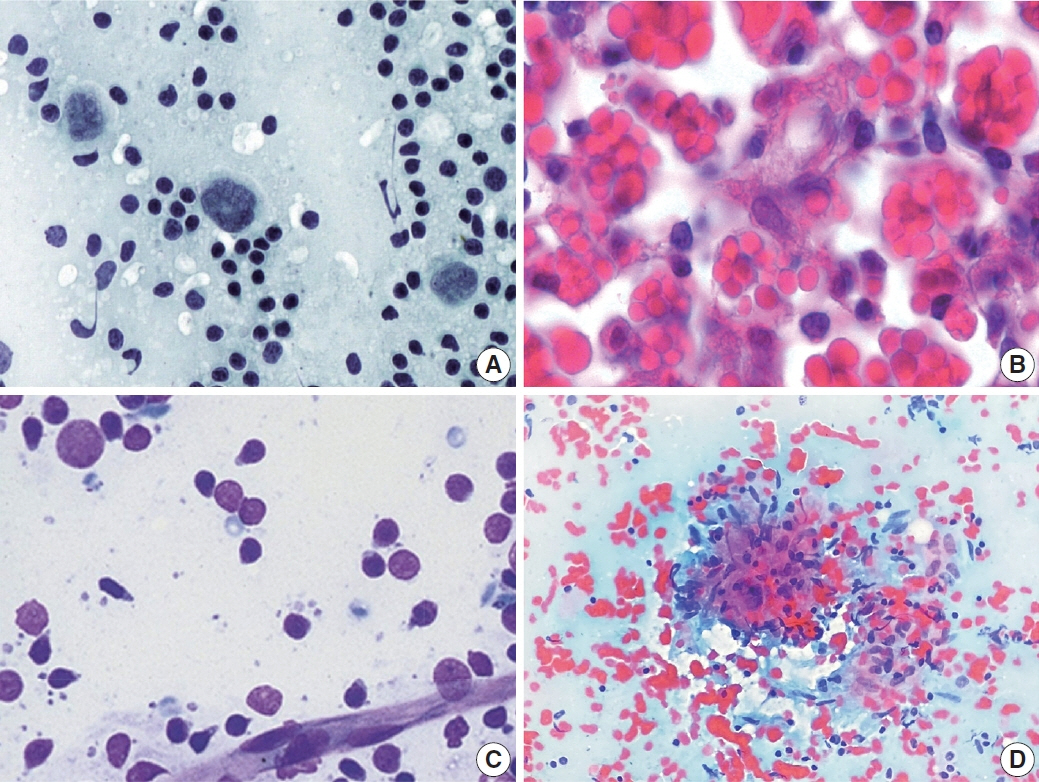
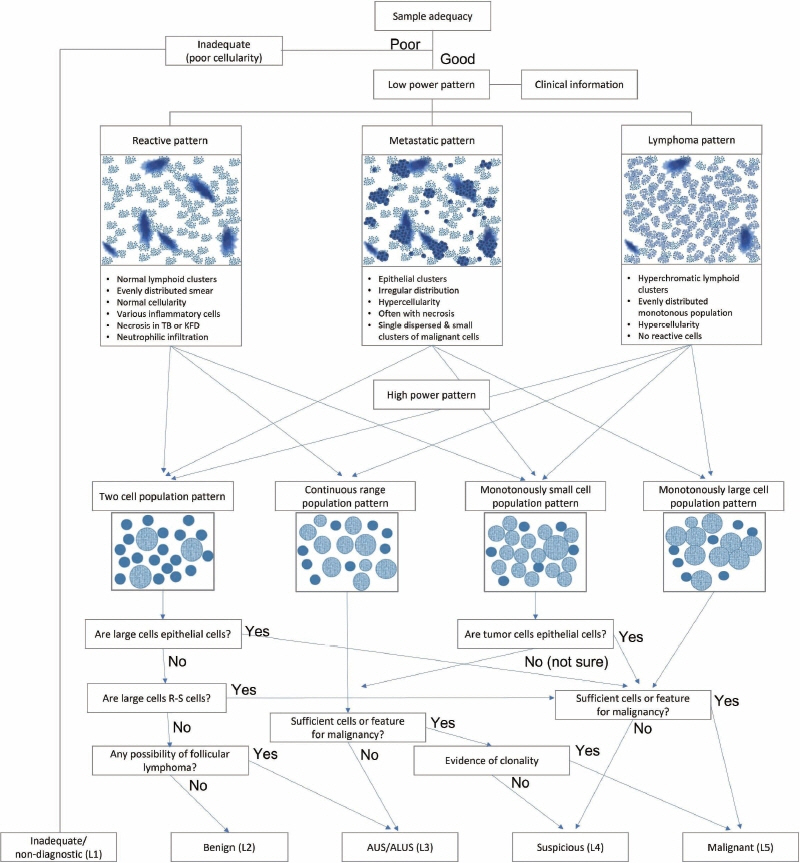
- The cytological diagnosis of lymph node lesions is extremely challenging because of the diverse diseases that cause lymph node enlargement, including both benign and malignant or metastatic lymphoid lesions. Furthermore, the cytological findings of different lesions often resemble one another. A stepwise diagnostic approach is essential for a comprehensive diagnosis that combines: clinical findings, including age, sex, site, multiplicity, and ultrasonography findings; low-power reactive, metastatic, and lymphoma patterns; high-power population patterns, including two populations of continuous range, small monotonous pattern and large monotonous pattern; and disease-specific diagnostic clues including granulomas and lymphoglandular granules. It is also important to remember the histological features of each diagnostic category that are common in lymph node cytology and to compare them with cytological findings. It is also essential to identify a few categories of diagnostic pitfalls that often resemble lymphomas and easily lead to misdiagnosis, particularly in malignant small round cell tumors, poorly differentiated squamous cell carcinomas, and nasopharyngeal undifferentiated carcinoma. Herein, we review a stepwise approach for fine needle aspiration cytology of lymphoid diseases and suggest a diagnostic algorithm that uses this approach and the Sydney classification system.
Figure
Reference
-
References
1. Zhou J, Li F, Meng L, et al. Fine needle aspiration cytology for lymph nodes: a three-year study. Br J Biomed Sci. 2016; 73:28–31.2. Cibas ES, Ducatman BS. Cytology: diagnostic principles and clinical correlates. 5th ed. Maryland Heights: Elsevier Inc;2020.3. Rammeh S, Romdhane E, Sassi A, et al. Accuracy of fine-needle aspiration cytology of head and neck masses. Diagn Cytopathol. 2019; 47:394–9.4. Houcine Y, Romdhane E, Blel A, et al. Evaluation of fine needle aspiration cytology in the diagnosis of cervical lymph node lymphomas. J Craniomaxillofac Surg. 2018; 46:1117–20.5. Rammeh S, Ben Rejeb H, M’Farrej M K, et al. Cervical node fine needle aspiration: factors influencing the failure rate. Rev Stomatol Chir Maxillofac Chir Orale. 2014; 115:85–7.6. Heo I, Park S, Jung CW, et al. Fine needle aspiration cytology of parathyroid lesions. Korean J Pathol. 2013; 47:466–71.7. Agarwal AM, Bentz JS, Hungerford R, Abraham D. Parathyroid fine-needle aspiration cytology in the evaluation of parathyroid adenoma: cytologic findings from 53 patients. Diagn Cytopathol. 2009; 37:407–10.8. Gronkiewicz JJ, Vade A. Cervical lymph node fine needle aspiration in patients with no history of malignancy. Ultrasound Q. 2013; 29:323–6.9. Hong SA, Jung H, Kim SS, et al. Current status of cytopathology practice in Korea: impact of the coronavirus pandemic on cytopathology practice. J Pathol Transl Med. 2022; 56:361–9.10. Al-Abbadi MA, Barroca H, Bode-Lesniewska B, et al. A proposal for the performance, classification, and reporting of lymph node fine-needle aspiration cytopathology: the Sydney system. Acta Cytol. 2020; 64:306–22.11. Duraiswami R, Margam S, Chandran P, Prakash A. Spectrum of pathologies on FNAC evaluation of peripheral lymph nodes at a tertiary care center in hyderabad: a retrospective study. Int J Adv Med. 2017; 4:27–33.12. Cho J. Basic immunohistochemistry for lymphoma diagnosis. Blood Res. 2022; 57:55–61.13. Kuppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol. 2005; 37:511–7.14. Jaffe ES, Nicolae A, Pittaluga S. Peripheral T-cell and NK-cell lymphomas in the WHO classification: pearls and pitfalls. Mod Pathol. 2013; 26 Suppl 1:S71–87.15. de Leval L, Savilo E, Longtine J, Ferry JA, Harris NL. Peripheral Tcell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am J Surg Pathol. 2001; 25:395–400.16. Huang Y, Moreau A, Dupuis J, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009; 33:682–90.17. Dobay MP, Lemonnier F, Missiaglia E, et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica. 2017; 102:e148–51.18. Rizzo K, Nassiri M. Diagnostic workup of small B cell lymphomas: a laboratory perspective. Lymphoma. 2012; 2012:346084.19. Jacobs JC, Katz RL, Shabb N, el-Naggar A, Ordonez NG, Pugh W. Fine needle aspiration of lymphoblastic lymphoma: a multiparameter diagnostic approach. Acta Cytol. 1992; 36:887–94.20. Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ. Diffuse large Bcell lymphoma variants: an update. Pathology. 2020; 52:53–67.21. el-Okda M, Hyeh Y, Xie SS, Hsu SM. Russell bodies consist of heterogenous glycoproteins in B-cell lymphoma cells. Am J Clin Pathol. 1992; 97:866–71.22. Francis IM, Das DK, al-Rubah NA, Gupta SK. Lymphoglandular bodies in lymphoid lesions and non-lymphoid round cell tumours: a quantitative assessment. Diagn Cytopathol. 1994; 11:23–7.23. Jimenez-Heffernan JA, Diaz Del Arco C, Adrados M. A cytological review of follicular dendritic cell-derived tumors with emphasis on follicular dendritic cell sarcoma and unicentric Castleman disease. Diagnostics (Basel). 2022; 12:406.24. Gupta P, Gupta N, Kumar P, et al. Assessment of risk of malignancy by application of the proposed Sydney system for classification and reporting lymph node cytopathology. Cancer Cytopathol. 2021; 129:701–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Ultrasound-Guided Fine Needle Aspiration in Breast Lesions
- Fine Needle Aspiration Cytology of Langerhans' Cell Histiocytosis in the Lymph Node
- Comment on “A stepwise approach to fine needle aspiration cytology of lymph nodes”
- Fine Needle Aspiration Cytology of Metastatic Seminoma in Cervical Lymph Node: A Case Report
- Fine Needle Aspiration Cytology of Subacute Necrotizing Lymphadenitis: Three Cases Report