Korean J Pain.
2023 Jul;36(3):316-327. 10.3344/kjp.22398.
Antisense oligodeoxynucleotides against dynamin-related protein 1 reduce remifentanilinduced hyperalgesia by modulating spinal N-methyl-D-aspartate receptor expression in rats
- Affiliations
-
- 1Department of Anesthesiology, 1st Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Operative Room Nursing, 1st Affiliated Hospital, Wenzhou Medical University, Wenzhou, Zhejiang, China
- KMID: 2544243
- DOI: http://doi.org/10.3344/kjp.22398
Abstract
- Background
Spinal N-methyl-D-aspartate (NMDA) receptor activation is attributed to remifentanil-induced hyperalgesia (RIH). However, the specific mechanism and subsequent treatment is still unknown. Previous studies have shown that the dynamin-related protein 1 (DRP1)-mitochondria-reactive oxygen species (ROS) pathway plays an important role in neuropathic pain. This study examined whether antisense oligodeoxynucleotides against DRP1 (AS-DRP1) could reverse RIH.
Methods
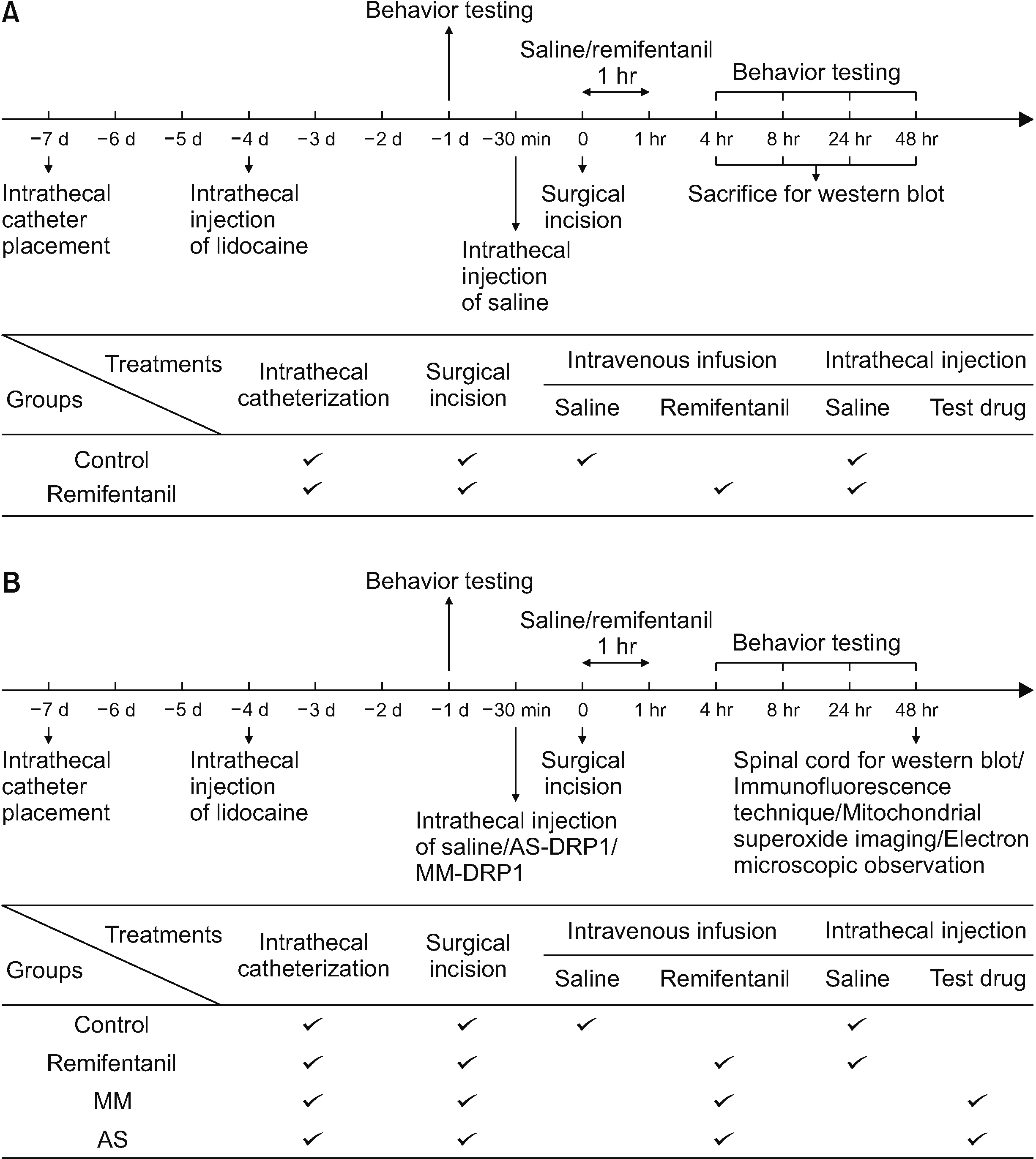
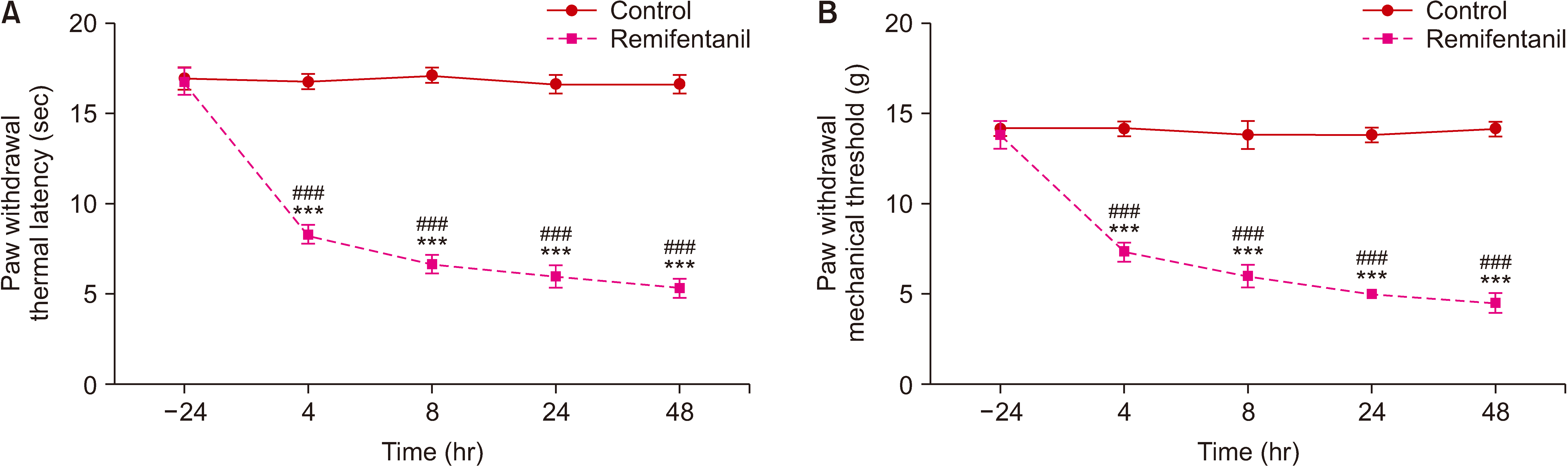
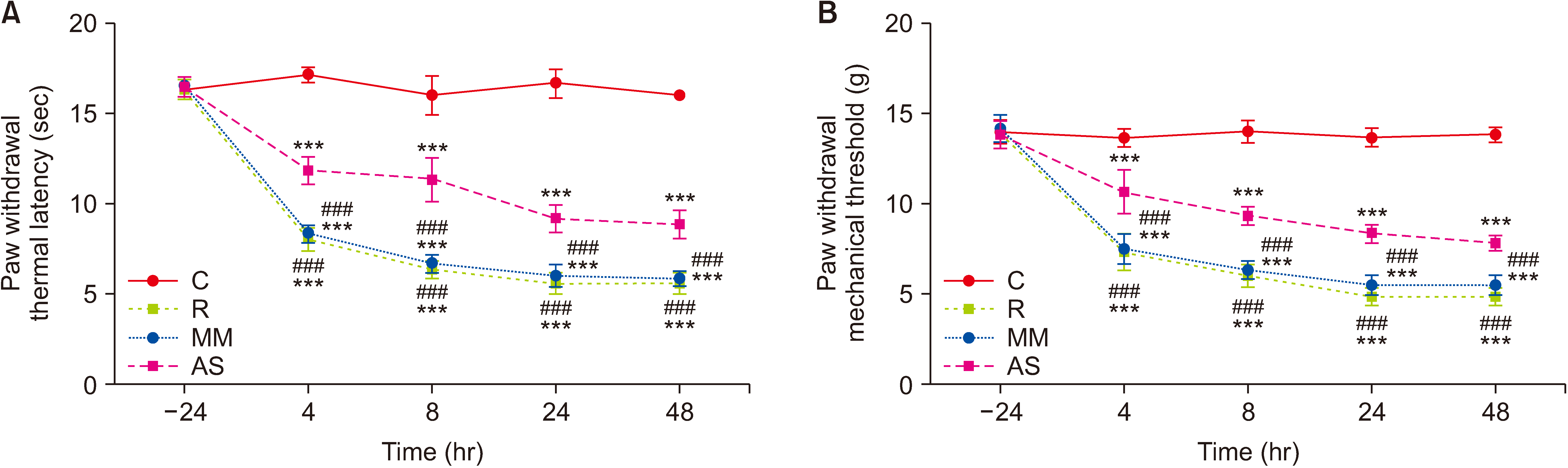
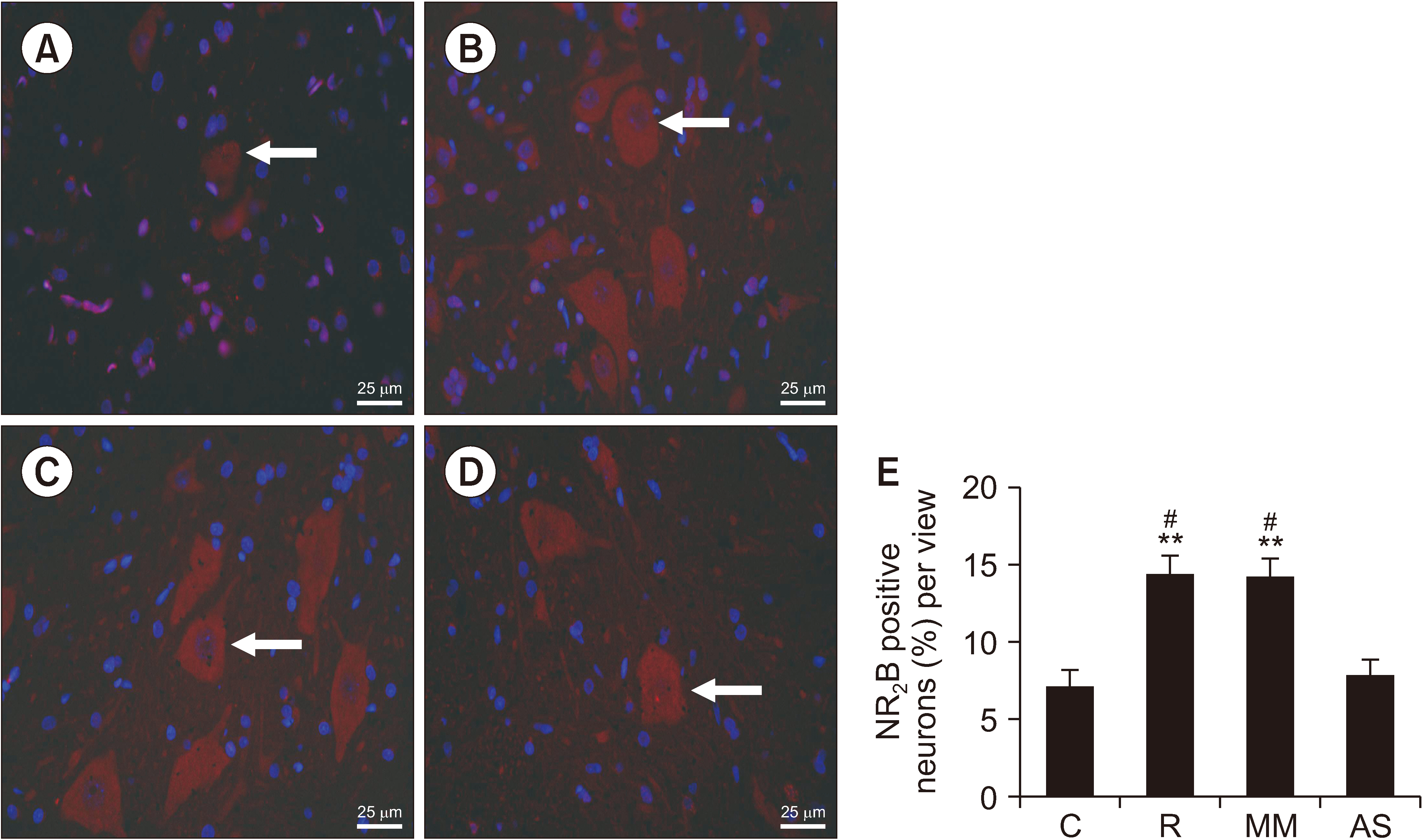
The authors first measured changes in paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL) at 24 hours before remifentanil infusion and 4, 8, 24, and 48 hours after infusion. The expression levels of DRP1 and NR2B were measured after behavioral testing using Western blotting. In addition, DRP1 expression was knocked down by intrathecal administration of AS-DRP1 to investigate the effects of DRP1 on RIH. The behavioral testing, the expression levels of spinal DRP1 and NR2B, and dorsal mitochondrial superoxide were measured. Changes in mitochondrial morphology were assessed using electron microscopy.
Results
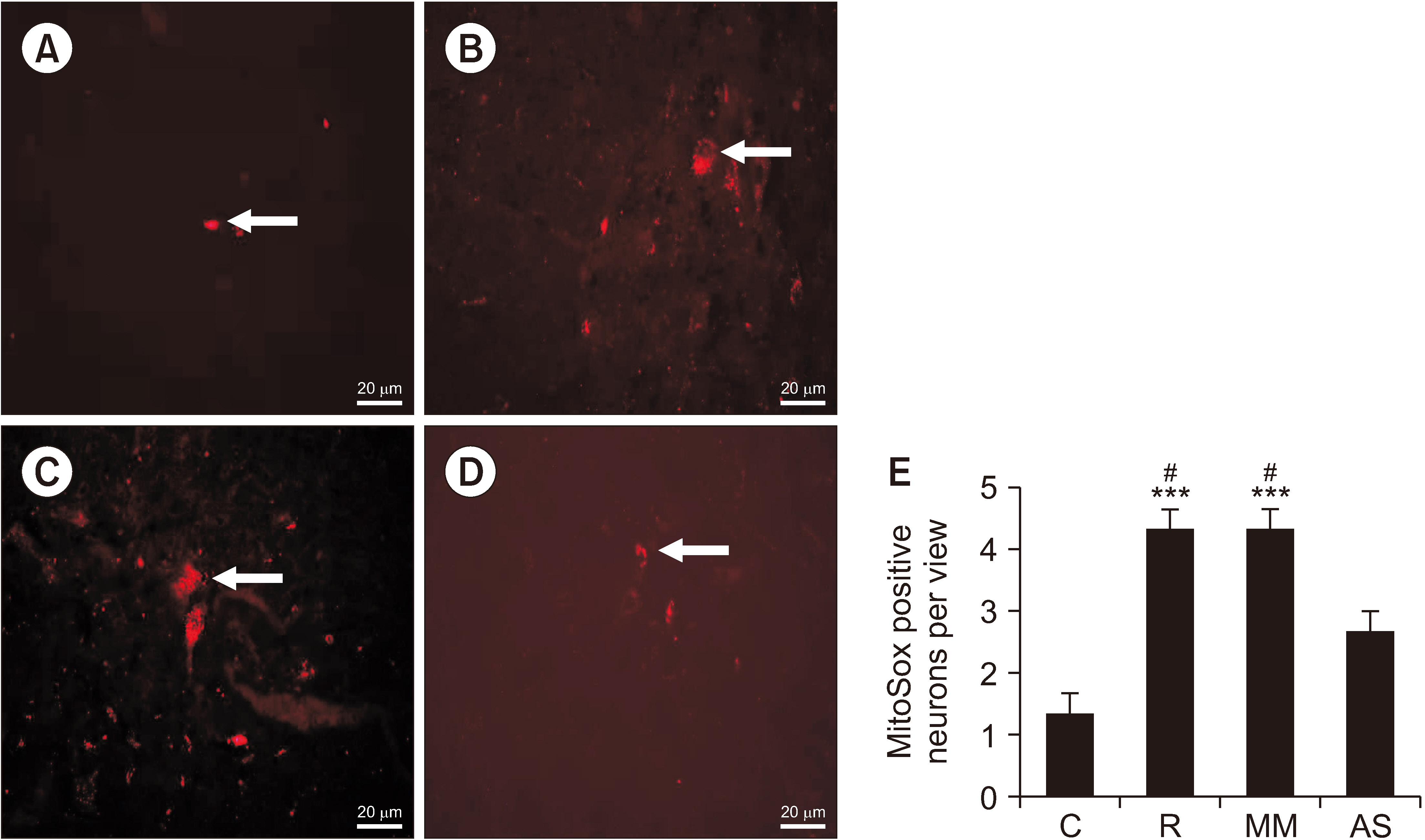
After remifentanil exposure, upregulation of spinal DRP1 and NR2B was observed along with a reduction in PWMT and PWTL. In addition, AS-DRP1 improved RIH-induced PWTL and PWMT (P < 0.001 and P < 0.001) and reduced remifentanil-mediated enhancement of spinal DRP1 and NR2B expression (P = 0.020 and P = 0.022). More importantly, AS-DRP1 reversed RIH-induced mitochondrial fission (P = 0.020) and mitochondrial superoxide upregulation (P = 0.031).
Conclusions
These results indicate that AS-DRP1 could modulate NMDA receptor expression to prevent RIH through the DRP1-mitochondria-ROS pathway.
Keyword
Figure
Cited by 1 articles
-
Towards precision pain management—the case for targeting DRP1 in remifentanil-induced hyperalgesia
Ki Tae Jung
Korean J Pain. 2023;36(4):405-407. doi: 10.3344/kjp.23258.
Reference
-
1. Kim SH, Stoicea N, Soghomonyan S, Bergese SD. 2014; Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 5:108. DOI: 10.3389/fphar.2014.00108. PMID: 24847273. PMCID: PMC4021143. PMID: b24749088e62475092776aa8e082e248.
Article2. Lee C, Song YK, Lee JH, Ha SM. 2011; The effects of intraoperative adenosine infusion on acute opioid tolerance and opioid induced hyperalgesia induced by remifentanil in adult patients undergoing tonsillectomy. Korean J Pain. 24:7–12. DOI: 10.3344/kjp.2011.24.1.7. PMID: 21390173. PMCID: PMC3049980.
Article3. Shu RC, Zhang LL, Wang CY, Li N, Wang HY, Xie KL, et al. 2015; Spinal peroxynitrite contributes to remifentanil-induced postoperative hyperalgesia via enhancement of divalent metal transporter 1 without iron-responsive element-mediated iron accumulation in rats. Anesthesiology. 122:908–20. DOI: 10.1097/ALN.0000000000000562. PMID: 25501899.4. Roeckel LA, Le Coz GM, Gavériaux-Ruff C, Simonin F. 2016; Opioid-induced hyperalgesia: cellular and molecular mechanisms. Neuroscience. 338:160–82. DOI: 10.1016/j.neuroscience.2016.06.029. PMID: 27346146.
Article5. Zhang L, Shu R, Zhao Q, Li Y, Yu Y, Wang G. 2016; Preoperative butorphanol and flurbiprofen axetil therapy attenuates remifentanil-induced hyperalgesia after laparoscopic gynaecological surgery: a randomized double-blind controlled trial. Br J Anaesth. 117:504–11. DOI: 10.1093/bja/aew248. PMID: 28077539.
Article6. Zhao M, Joo DT. 2008; Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 109:308–17. DOI: 10.1097/ALN.0b013e31817f4c5d. PMID: 18648240.7. Li Y, Wang H, Xie K, Wang C, Yang Z, Yu Y, et al. 2013; Inhibition of glycogen synthase kinase-3β prevents remifentanil-induced hyperalgesia via regulating the expression and function of spinal N-methyl-D-aspartate receptors in vivo and vitro. PLoS One. 8:e77790. DOI: 10.1371/journal.pone.0077790. PMID: 24147079. PMCID: PMC3797695. PMID: a76a2e1e9d0f4f6ea7bffc9e6b87bdd7.
Article8. Wang C, Li Y, Wang H, Xie K, Shu R, Zhang L, et al. 2015; Inhibition of DOR prevents remifentanil induced postoperative hyperalgesia through regulating the trafficking and function of spinal NMDA receptors in vivo and in vitro. Brain Res Bull. 110:30–9. DOI: 10.1016/j.brainresbull.2014.12.001. PMID: 25498394.
Article9. Dikalov SI, Harrison DG. 2014; Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal. 20:372–82. DOI: 10.1089/ars.2012.4886. PMID: 22978713. PMCID: PMC3887411.
Article10. Areti A, Yerra VG, Komirishetty P, Kumar A. 2016; Potential therapeutic benefits of maintaining mitochondrial health in peripheral neuropathies. Curr Neuropharmacol. 14:593–609. DOI: 10.2174/1570159X14666151126215358. PMID: 26818748. PMCID: PMC4981743.
Article11. Im YB, Jee MK, Choi JI, Cho HT, Kwon OH, Kang SK. 2012; Molecular targeting of NOX4 for neuropathic pain after traumatic injury of the spinal cord. Cell Death Dis. 3:e426. DOI: 10.1038/cddis.2012.168. PMID: 23152062. PMCID: PMC3542604.
Article12. Gwak YS, Hassler SE, Hulsebosch CE. 2013; Reactive oxygen species contribute to neuropathic pain and locomotor dysfunction via activation of CamKII in remote segments following spinal cord contusion injury in rats. Pain. 154:1699–708. DOI: 10.1016/j.pain.2013.05.018. PMID: 23707296.
Article13. Salvemini D, Little JW, Doyle T, Neumann WL. 2011; Roles of reactive oxygen and nitrogen species in pain. Free Radic Biol Med. 51:951–66. DOI: 10.1016/j.freeradbiomed.2011.01.026. PMID: 21277369. PMCID: PMC3134634.
Article14. Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, et al. 2009; Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 164:702–10. DOI: 10.1016/j.neuroscience.2009.07.019. PMID: 19607887. PMCID: PMC2860430.
Article15. Amigo I, da Cunha FM, Forni MF, Garcia-Neto W, Kakimoto PA, Luévano-Martínez LA, et al. 2016; Mitochondrial form, function and signalling in aging. Biochem J. 473:3421–49. DOI: 10.1042/BCJ20160451. PMID: 27729586.
Article16. Manczak M, Kandimalla R, Yin X, Reddy PH. 2019; Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum Mol Genet. 28:177–99. Erratum in: Hum Mol Genet 2019; 28: 875-6. DOI: 10.1093/hmg/ddy399. PMID: 30452626. PMCID: PMC6381310.
Article17. Smirnova E, Griparic L, Shurland DL, van der Bliek AM. 2001; Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 12:2245–56. DOI: 10.1091/mbc.12.8.2245. PMID: 11514614. PMCID: PMC58592.18. Ye L, Xiao L, Bai X, Yang SY, Li Y, Chen Y, et al. 2016; Spinal mitochondrial-derived ROS contributes to remifentanil-induced postoperative hyperalgesia via modulating NMDA receptor in rats. Neurosci Lett. 634:79–86. DOI: 10.1016/j.neulet.2016.09.016. PMID: 27637388.
Article19. Liu X, Zhang J, Zhao H, Mei H, Lian Q, ShangGuan W. 2014; The effect of propofol on intrathecal morphine-induced pruritus and its mechanism. Anesth Analg. 118:303–9. DOI: 10.1213/ANE.0000000000000086. PMID: 24445631.
Article20. Gao Y, Zhan W, Jin Y, Chen X, Cai J, Zhou X, et al. 2022; KCC2 receptor upregulation potentiates antinociceptive effect of GABAAR agonist on remifentanil-induced hyperalgesia. Mol Pain. 18:17448069221082880. DOI: 10.1177/17448069221082880. PMID: 35352582. PMCID: PMC8972932.
Article21. Gao Y, Zhou S, Pan Y, Gu L, He Y, Sun J. 2020; Wnt3a inhibitor attenuates remifentanil-induced hyperalgesia via downregulating spinal NMDA receptor in rats. J Pain Res. 13:1049–58. DOI: 10.2147/JPR.S250663. PMID: 32547170. PMCID: PMC7245459.22. Brennan TJ, Vandermeulen EP, Gebhart GF. 1996; Characterization of a rat model of incisional pain. Pain. 64:493–502. DOI: 10.1016/0304-3959(95)01441-1. PMID: 8783314.
Article23. Kanda H, Liu S, Iida T, Yi H, Huang W, Levitt RC, et al. 2016; Inhibition of mitochondrial fission protein reduced mechanical allodynia and suppressed spinal mitochondrial superoxide induced by perineural human immunodeficiency virus gp120 in Rats. Anesth Analg. 122:264–72. DOI: 10.1213/ANE.0000000000000962. PMID: 26418124.
Article24. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. 1994; Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63. DOI: 10.1016/0165-0270(94)90144-9. PMID: 7990513.
Article25. Wang G, Yang Y, Wang C, Huang J, Wang X, Liu Y, et al. 2020; Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats. Korean J Pain. 33:216–25. DOI: 10.3344/kjp.2020.33.3.216. PMID: 32606266. PMCID: PMC7336342.
Article26. Cabañero D, Campillo A, Célérier E, Romero A, Puig MM. 2009; Pronociceptive effects of remifentanil in a mouse model of postsurgical pain: effect of a second surgery. Anesthesiology. 111:1334–45. DOI: 10.1097/ALN.0b013e3181bfab61. PMID: 19934880.27. Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, et al. 2009; Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 29:159–68. DOI: 10.1523/JNEUROSCI.3792-08.2009. PMID: 19129394. PMCID: PMC2757011.
Article28. Schwartz ES, Lee I, Chung K, Chung JM. 2008; Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 138:514–24. DOI: 10.1016/j.pain.2008.01.029. PMID: 18375065. PMCID: PMC2581506.
Article29. Kun L, Lu L, Yongda L, Xingyue L, Guang H. 2019; Hyperbaric oxygen promotes mitophagy by activating CaMKKβ/AMPK signal pathway in rats of neuropathic pain. Mol Pain. 15:1744806919871381. DOI: 10.1177/1744806919871381. PMID: 31382832. PMCID: PMC6710678.30. Lenz H, Raeder J, Draegni T, Heyerdahl F, Schmelz M, Stubhaug A. 2011; Effects of COX inhibition on experimental pain and hyperalgesia during and after remifentanil infusion in humans. Pain. 152:1289–97. DOI: 10.1016/j.pain.2011.02.007. PMID: 21396775.
Article31. Deng M, Chen SR, Pan HL. 2019; Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol Life Sci. 76:1889–99. DOI: 10.1007/s00018-019-03047-y. PMID: 30788514. PMCID: PMC6482077.
Article32. Qi F, Liu T, Zhang X, Gao X, Li Z, Chen L, et al. 2020; Ketamine reduces remifentanil-induced postoperative hyperalgesia mediated by CaMKII-NMDAR in the primary somatosensory cerebral cortex region in mice. Neuropharmacology. 162:107783. DOI: 10.1016/j.neuropharm.2019.107783. PMID: 31541650.
Article33. Hahnenkamp K, Nollet J, Van Aken HK, Buerkle H, Halene T, Schauerte S, et al. 2004; Remifentanil directly activates human N-methyl-D-aspartate receptors expressed in Xenopus laevis oocytes. Anesthesiology. 100:1531–7. DOI: 10.1097/00000542-200406000-00028. PMID: 15166575.
Article34. Tsushima K, Bugger H, Wende AR, Soto J, Jenson GA, Tor AR, et al. 2018; Mitochondrial reactive oxygen species in lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ Res. 122:58–73. DOI: 10.1161/CIRCRESAHA.117.311307. PMID: 29092894. PMCID: PMC5756120.
Article35. Jenner P. 1994; Oxidative damage in neurodegenerative disease. Lancet. 344:796–8. DOI: 10.1016/S0140-6736(94)92347-7. PMID: 7916079.
Article36. Bailey SM. 2003; A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic Res. 37:585–96. DOI: 10.1080/1071576031000091711. PMID: 12868485.37. Gibellini L, Bianchini E, De Biasi S, Nasi M, Cossarizza A, Pinti M. 2015; Natural compounds modulating mitochondrial functions. Evid Based Complement Alternat Med. 2015:527209. DOI: 10.1155/2015/527209. PMID: 26167193. PMCID: PMC4489008.
Article38. Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD. 2011; Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci. 31:11404–10. DOI: 10.1523/JNEUROSCI.2223-11.2011. PMID: 21813700. PMCID: PMC3157245.
Article39. Hao M, Tang Q, Wang B, Li Y, Ding J, Li M, et al. 2020; Resveratrol suppresses bone cancer pain in rats by attenuating inflammatory responses through the AMPK/Drp1 signaling. Acta Biochim Biophys Sin (Shanghai). 52:231–40. DOI: 10.1093/abbs/gmz162. PMID: 32072182.
Article40. Li MY, Ding JQ, Tang Q, Hao MM, Wang BH, Wu J, et al. 2019; SIRT1 activation by SRT1720 attenuates bone cancer pain via preventing Drp1-mediated mitochondrial fission. Biochim Biophys Acta Mol Basis Dis. 1865:587–98. DOI: 10.1016/j.bbadis.2018.12.017. PMID: 30579931.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Nociceptive Responses in Streptozotocin-Induced Diabetic Rats and the Related Expression of Spinal NR2B Subunit of N-Methyl-D-Aspartate Receptors
- Antiallodynic Effects of Electroacupuncture Combined with MK-801 Treatment through the Regulation of p35/p25 in Experimental Diabetic Neuropathy
- The Effect of Intrathecal NMDA and non-NMDA Receptor Antagonist on the Hyperalgesia Observed after Thermal Injury in the Rat
- Opioid-induced hyperalgesia
- The Effect of Intrathecal ACEA 1021, Competitive Glycine-site NMDA Receptor Antagonist on the Hyperalgesia Observed after Thermal Injury in the Rat