Cancer Res Treat.
2023 Jul;55(3):1048-1052. 10.4143/crt.2022.1529.
Efficacy of Olaparib in Treatment-Refractory, Metastatic Breast Cancer with Uncommon Somatic BRCA Mutations Detected in Circulating Tumor DNA
- Affiliations
-
- 1Division of Pulmonary, and Critical Care Medicine, Department of Medicine, Stanford University, Stanford, CA, USA
- 2Department of Chemistry, Yonsei University, Seoul, Korea
- 3Department of Genomic Medicine, Seoul National University Hospital, Seoul, Korea
- 4IMBdx, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 6Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Cancer Research Institute, Seoul National University, Seoul, Korea
- KMID: 2544184
- DOI: http://doi.org/10.4143/crt.2022.1529
Abstract
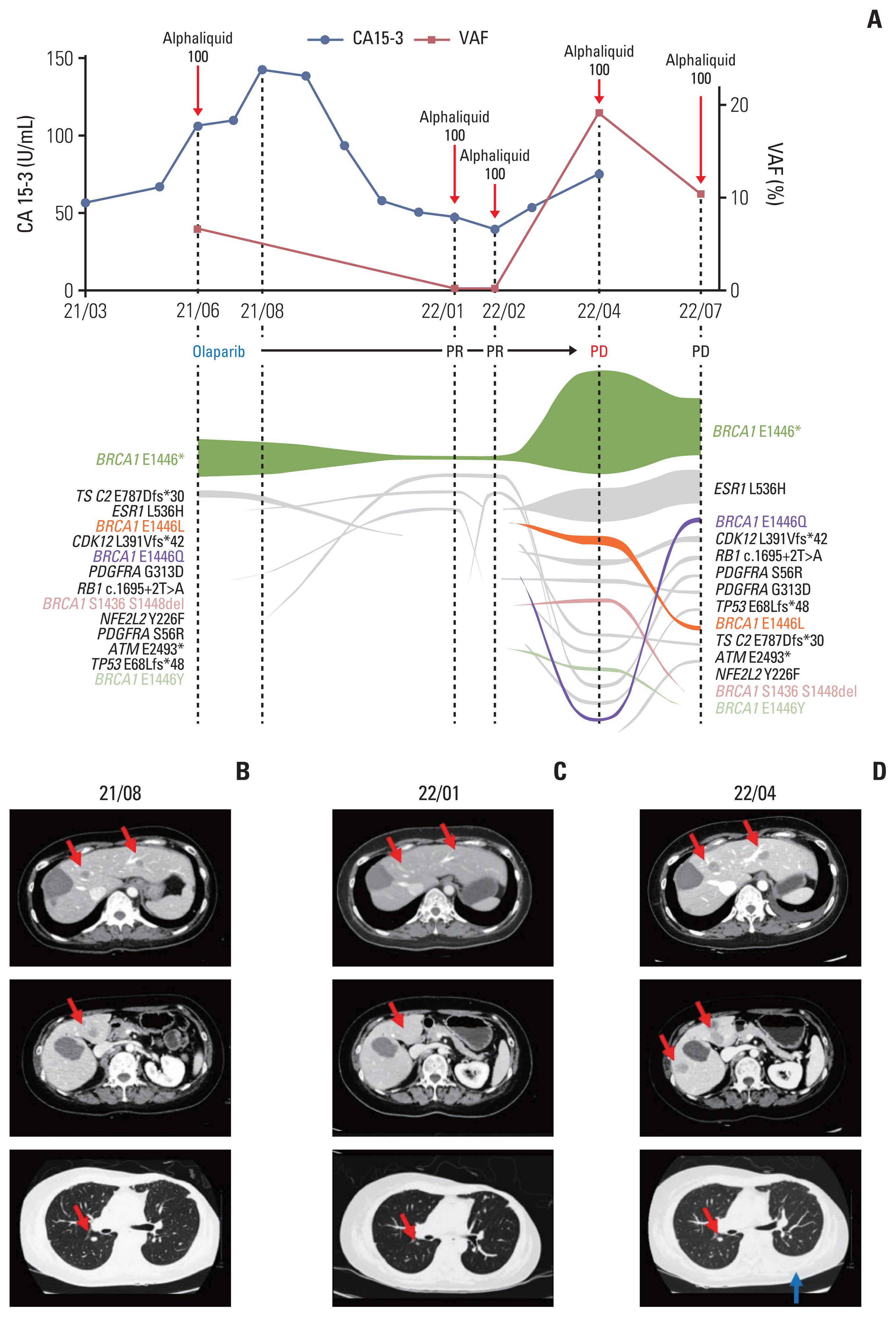
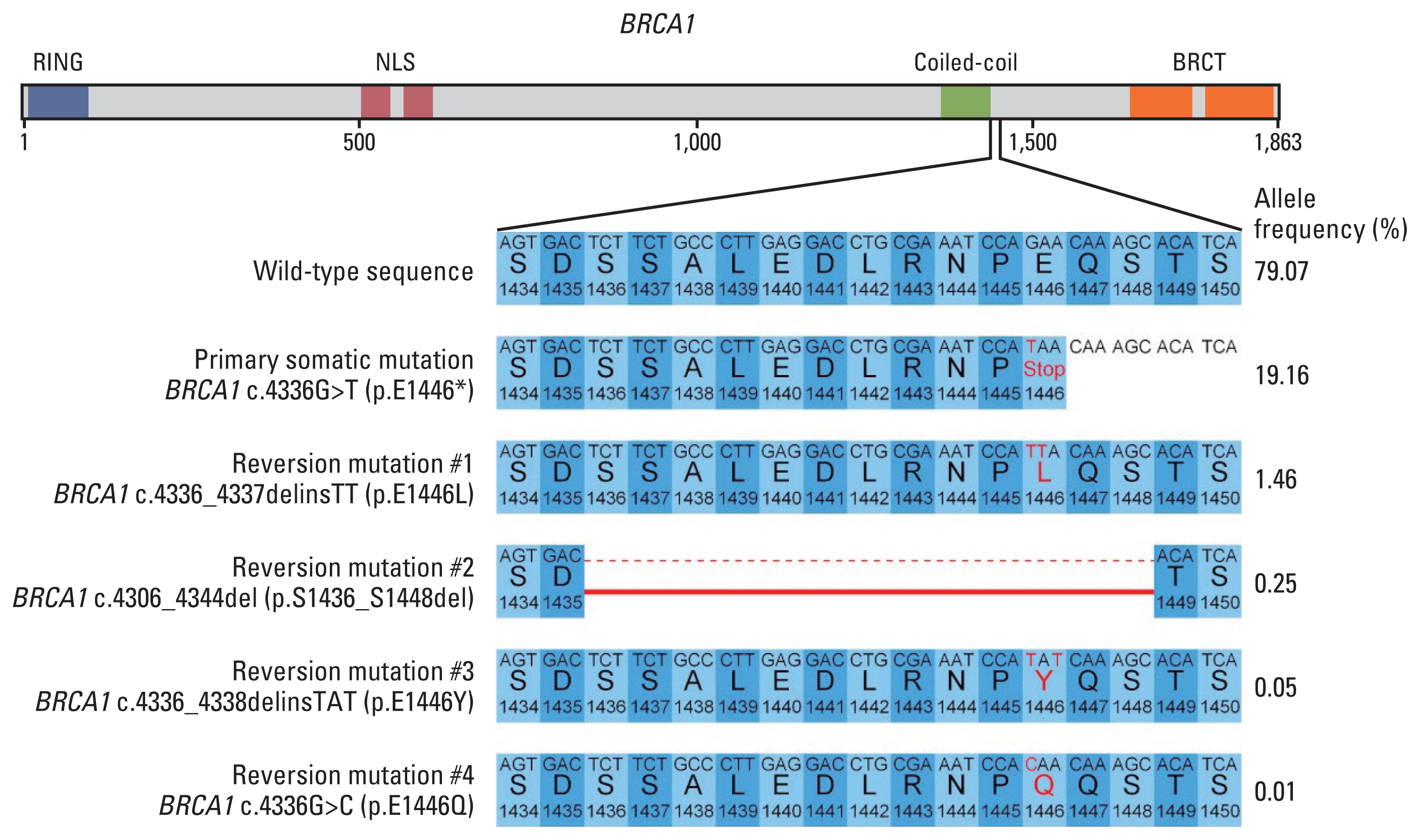
- Poly(ADP-ribose) polymerase inhibitors have been shown dramatic responses in patients with BRCAness. However, clinical studies have been limited to breast cancer patients with germline mutations. Here, we describe a patient with metastatic breast cancer who had a rare BRCA1 somatic mutation (BRCA1 c.4336G>T (p.E1446*)) detected by cell-free DNA analysis after failing standard therapies. This tier III variant of unknown significance was predicted to be a pathogenic variant in our assessment, leading us to consider off-label treatment with olaparib. The patient responded well to olaparib for several months, with a decrease in allele frequency of this BRCA1 somatic mutation in cell-free DNA. Olaparib resistance subsequently developed with an increase in the allele frequency and new BRCA1 reversion mutations. To our knowledge, this is the first report confirming BRCA1 c.4336G>T (p.E1446*) as a mutation sensitive to olaparib in breast cancer and describing the dynamic changes in the associated mutations using liquid biopsy.
Figure
Reference
-
References
1. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.
Article2. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.
Article3. Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020; 38:4274–82.
Article4. Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017; 23:703–13.5. Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019; 9:210–9.6. Li H, Liu ZY, Wu N, Chen YC, Cheng Q, Wang J. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer. 2020; 19:107.
Article7. Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019; 47:D886–D94.
Article8. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021; 23:85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- BRCA1 Reversion Mutation Confers Resistance to Olaparib and Camrelizumab in a Patient with Breast Cancer Liver Metastasis
- Olaparib plus bevacizumab as a first-line maintenance treatment for patients with advanced ovarian cancer by molecular status: an updated PAOLA-1 based cost-effectiveness analysis
- Analysis of Circulating Tumor DNA to Predict Neoadjuvant Therapy Effectiveness and Breast Cancer Recurrence
- Dual Disruption of DNA Repair by a Novel CHK2 Inhibitor, ART-446, and Olaparib is a Promising Strategy for Triple-Negative Breast Cancer Therapy
- Clinical Impact of Somatic Variants in Homologous RecombinationRepair-Related Genes in Ovarian High-Grade Serous Carcinoma