Cancer Res Treat.
2023 Jul;55(3):956-968. 10.4143/crt.2022.409.
Survival Benefit of Adjuvant Chemotherapy in Patients with Pancreatic Ductal Adenocarcinoma Who Underwent Surgery Following Neoadjuvant FOLFIRINOX
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Hematology and Oncology, Chungnam National University Hospital, Daejeon, Korea
- 4Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2544176
- DOI: http://doi.org/10.4143/crt.2022.409
Abstract
- Purpose
The benefit of adjuvant chemotherapy following curative-intent surgery in pancreatic ductal adenocarcinoma (PDAC) patients who had received neoadjuvant FOLFIRINOX is unclear. This study aimed to assess the survival benefit of adjuvant chemotherapy in this patient population.
Materials and Methods
This retrospective study included 218 patients with localized non-metastatic PDAC who received neoadjuvant FOLFIRINOX and underwent curative-intent surgery (R0 or R1) between January 2017 and December 2020. The association of adjuvant chemotherapy with disease-free survival (DFS) and overall survival (OS) was evaluated in overall patients and in the propensity score matched (PSM) cohort. Subgroup analysis was conducted according to the pathology-proven lymph node status.
Results
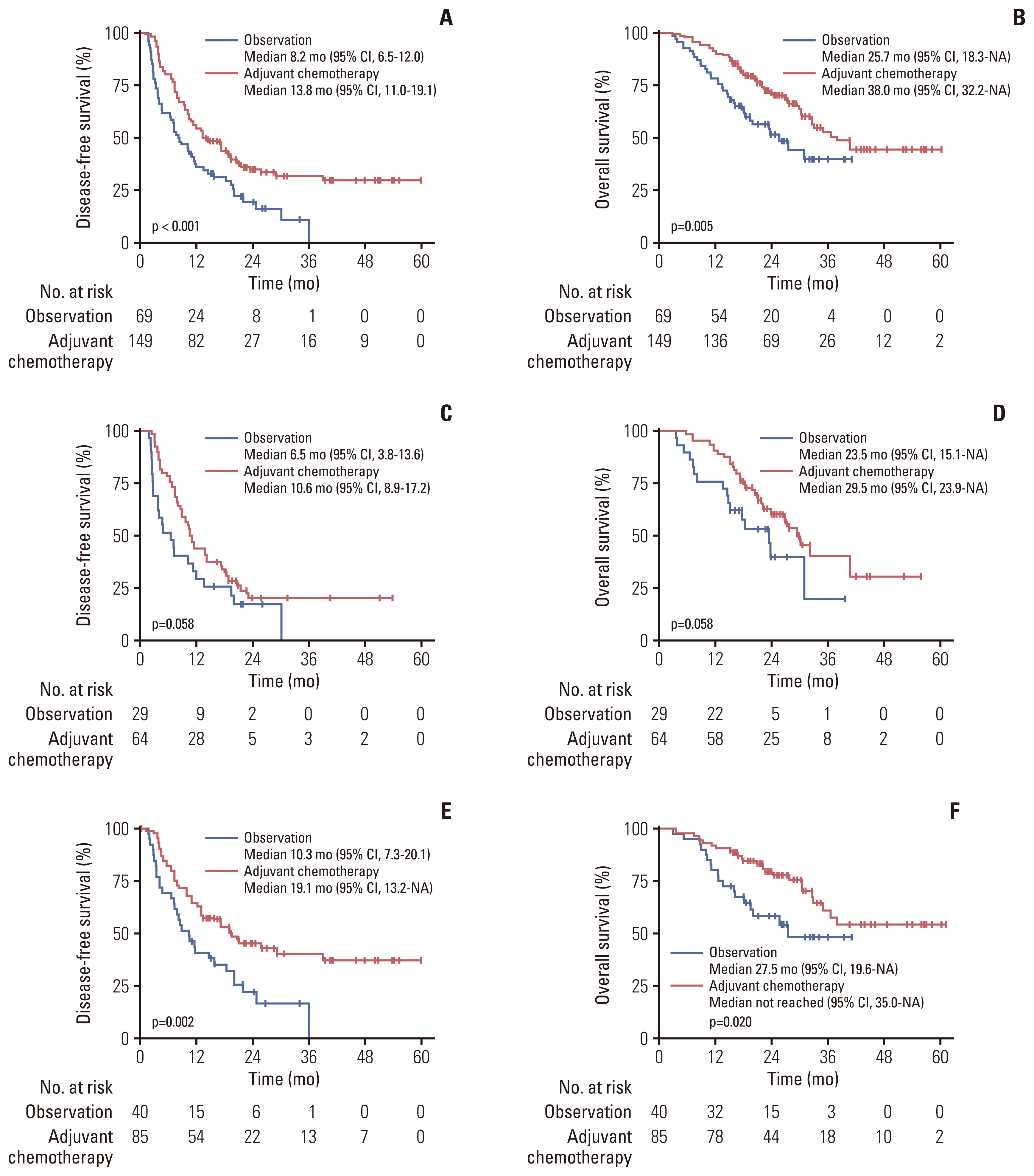
Adjuvant chemotherapy was administered to 149 patients (68.3%). In the overall cohort, the adjuvant chemotherapy group had significantly improved DFS and OS compared to the observation group (DFS: median, 13.8 months [95% confidence interval (CI), 11.0 to 19.1] vs. 8.2 months [95% CI, 6.5 to 12.0]; p < 0.001; and OS: median, 38.0 months [95% CI, 32.2 to not assessable] vs. 25.7 months [95% CI, 18.3 to not assessable]; p=0.005). In the PSM cohort of 57 matched pairs of patients, DFS and OS were better in the adjuvant chemotherapy group than in the observation group (p < 0.001 and p=0.038, respectively). In the multivariate analysis, adjuvant chemotherapy was a significant favorable prognostic factor (vs. observation; DFS: hazard ratio [HR], 0.51 [95% CI, 0.36 to 0.71; p < 0.001]; OS: HR, 0.45 [95% CI, 0.29 to 0.71; p < 0.001]).
Conclusion
Among PDAC patients who underwent surgery following neoadjuvant FOLFIRINOX, adjuvant chemotherapy may be associated with improved survival. Randomized studies should be conducted to validate this finding.
Keyword
Figure
Reference
-
References
1. Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018; 267:936–45.
Article2. Maeda S, Unno M, Yu J. Adjuvant and neoadjuvant therapy for pancreatic cancer. J Pancreatol. 2019; 2:100–6.3. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018; 24:4846–61.
Article4. Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goere D, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26(Suppl 5):v56–68.
Article5. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018; 379:2395–406.
Article6. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017; 389:1011–24.
Article7. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013; 310:1473–81.
Article8. Nagakawa Y, Sahara Y, Hosokawa Y, Murakami Y, Yamaue H, Satoi S, et al. Clinical impact of neoadjuvant chemotherapy and chemoradiotherapy in borderline resectable pancreatic cancer: analysis of 884 patients at facilities specializing in pancreatic surgery. Ann Surg Oncol. 2019; 26:1629–36.
Article9. Oba A, Ho F, Bao QR, Al-Musawi MH, Schulick RD, Del Chiaro M. Neoadjuvant treatment in pancreatic cancer. Front Oncol. 2020; 10:245.
Article10. Unno M, Hata T, Motoi F. Long-term outcome following neoadjuvant therapy for resectable and borderline resectable pancreatic cancer compared to upfront surgery: a meta-analysis of comparative studies by intention-to-treat analysis. Surg Today. 2019; 49:295–9.
Article11. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018; 105:946–58.
Article12. Yoo C, Shin SH, Kim KP, Jeong JH, Chang HM, Kang JH, et al. Clinical outcomes of conversion surgery after neoadjuvant chemotherapy in patients with borderline resectable and locally advanced unresectable pancreatic cancer: a single-center, retrospective analysis. Cancers (Basel). 2019; 11:278.
Article13. Yoo C, Lee SS, Song KB, Jeong JH, Hyung J, Park DH, et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer. 2020; 123:362–8.
Article14. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016; 17:801–10.
Article15. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817–25.
Article16. Janssen QP, Buettner S, Suker M, Beumer BR, Addeo P, Bachellier P, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. 2019; 111:782–94.
Article17. Kamarajah SK, White SA, Naffouje SA, Salti GI, Dahdaleh F. Adjuvant chemotherapy associated with survival benefit following neoadjuvant chemotherapy and pancreatectomy for pancreatic ductal adenocarcinoma: a population-based cohort study. Ann Surg Oncol. 2021; 28:6790–802.
Article18. van Roessel S, van Veldhuisen E, Klompmaker S, Janssen QP, Abu Hilal M, Alseidi A, et al. Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020; 6:1733–40.
Article19. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017; 15:1028–61.
Article20. Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017; 265:185–91.
Article21. Washington K, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons P, et al. Protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas [Internet]. Northfield, IL: College of American Pathologists;c2012. [cited 2022 16 May]. Available from: https://cap.objects.frb.io/protocols/cp-pancreas-exocrine-2016-v3301.pdf.22. Roland CL, Katz MH, Tzeng CW, Lin H, Varadhachary GR, Shroff R, et al. The addition of postoperative chemotherapy is associated with improved survival in patients with pancreatic cancer treated with preoperative therapy. Ann Surg Oncol. 2015; 22(Suppl 3):S1221–8.
Article23. Olecki EJ, Stahl KA, Torres MB, Peng JS, Dixon M, Shen C, et al. Adjuvant chemotherapy after neoadjuvant chemotherapy for pancreatic cancer is associated with improved survival for patients with low-risk pathology. Ann Surg Oncol. 2021; 28:3111–22.
Article24. Barnes CA, Krepline AN, Aldakkak M, Clarke CN, Christians KK, Khan AH, et al. Is adjuvant therapy necessary for all patients with localized pancreatic cancer who have received neoadjuvant therapy? J Gastrointest Surg. 2017; 21:1793–803.25. Swords DS, Francis SR, Lloyd S, Garrido-Laguna I, Mulvihill SJ, Gruhl JD, et al. Lymph node ratio in pancreatic adenocarcinoma after preoperative chemotherapy vs. preoperative chemoradiation and its utility in decisions about postoperative chemotherapy. J Gastrointest Surg. 2019; 23:1401–13.
Article26. Carrato A, Pazo-Cid R, Macarulla T, Gallego J, Jiménez-Fonseca P, Rivera F, et al. Sequential nab-paclitaxel/gemcitabine followed by modified FOLFOX for first-line metastatic pancreatic cancer: The SEQUENCE trial. J Clil Oncol. 2022; 40:4022.
Article27. Rinaldi Y, Pointet AL, Khemissa Akouz F, Le Malicot K, Wahiba B, Louafi S, et al. Gemcitabine plus nab-paclitaxel until progression or alternating with FOLFIRI.3, as first-line treatment for patients with metastatic pancreatic adenocarcinoma: the Federation Francophone de Cancerologie Digestive-PRODIGE 37 randomised phase II study (FIRGEMAX). Eur J Cancer. 2020; 136:25–34.
Article28. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021; 273:341–9.
Article29. Garnier J, Robin F, Ewald J, Marchese U, Bergeat D, Boudjema K, et al. Pancreatectomy with vascular resection after neoadjuvant FOLFIRINOX: who survives more than a year after surgery? Ann Surg Oncol. 2021; 28:4625–34.30. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. Predictors of resectability and survival in patients eith borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 2019; 269:733–40.31. Yoo C, Hwang I, Song TJ, Lee SS, Jeong JH, Park DH, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol. 2020; 12:1758835920953294.32. Pietrasz D, Marthey L, Wagner M, Blanc JF, Laurent C, Turrini O, et al. Pathologic major response after FOLFIRINOX is prognostic for patients secondary resected for borderline or locally advanced pancreatic adenocarcinoma: an AGEO-FRENCH, prospective, multicentric cohort. Ann Surg Oncol. 2015; 22(Suppl 3):S1196–205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant and Adjuvant Treatments for Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma: The Current Status of Pancreatic Ductal Adenocarcinoma Treatment in Japan
- Updates of Chemotherapy for Pancreatic Cancer
- Collective review of pancreatic carcinosarcoma, a very rare pancreatic malignancy
- Selection of Optimal Adjuvant Chemotherapeutic Agents for Pancreatic Cancer Treatment
- Contemporary management of borderline resectable pancreatic ductal adenocarcinoma