Korean J Transplant.
2023 Jun;37(2):118-123. 10.4285/kjt.22.0055.
Primary adenocarcinoma with yolk sac differentiation in the transplant ureter and salvage of the transplant kidney: a rare case report
- Affiliations
-
- 1Department of Urology, Command Hospital (Southern Command), Pune, India
- 2Department of Pathology, Command Hospital (Southern Command), Pune, India
- 3Department of Nephrology, Command Hospital (Southern Command), Pune, India
- 4Department of General Surgery, Military Hospital, Kargil, India
- KMID: 2544116
- DOI: http://doi.org/10.4285/kjt.22.0055
Abstract
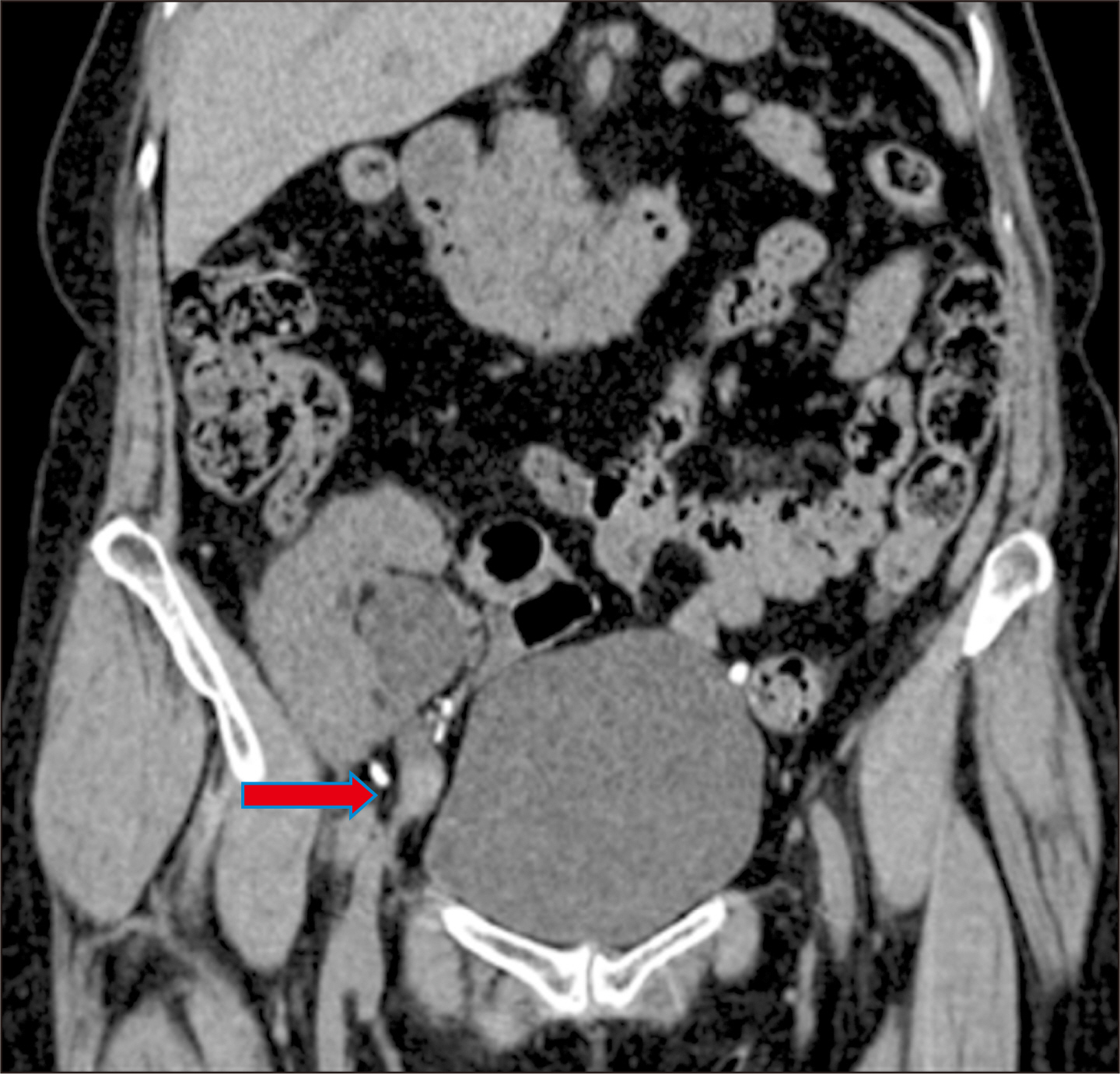
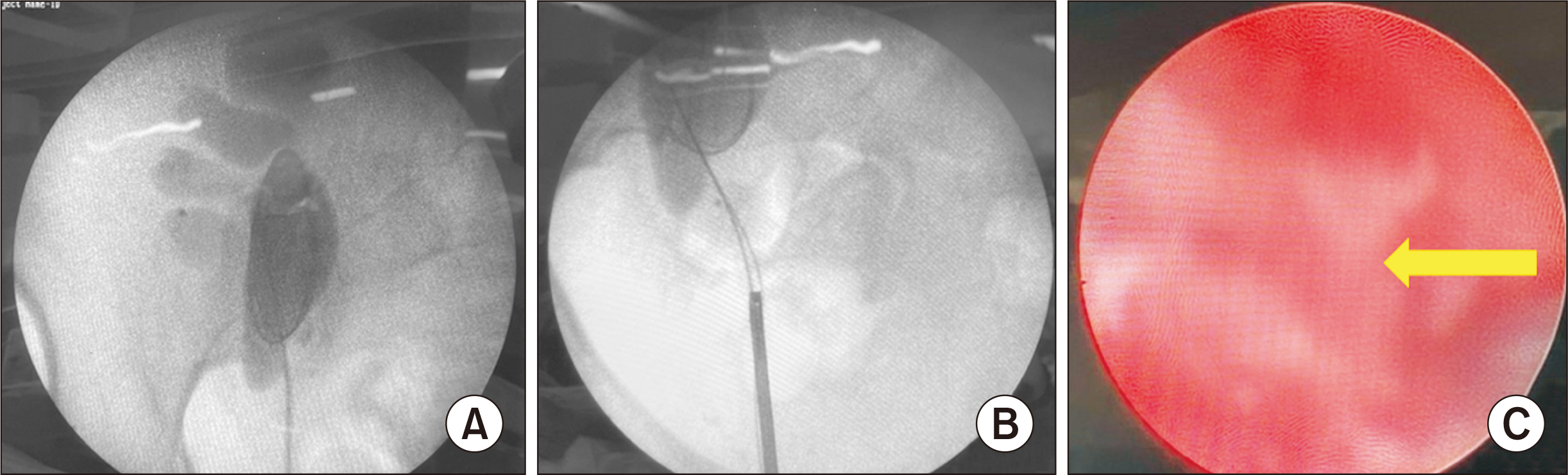
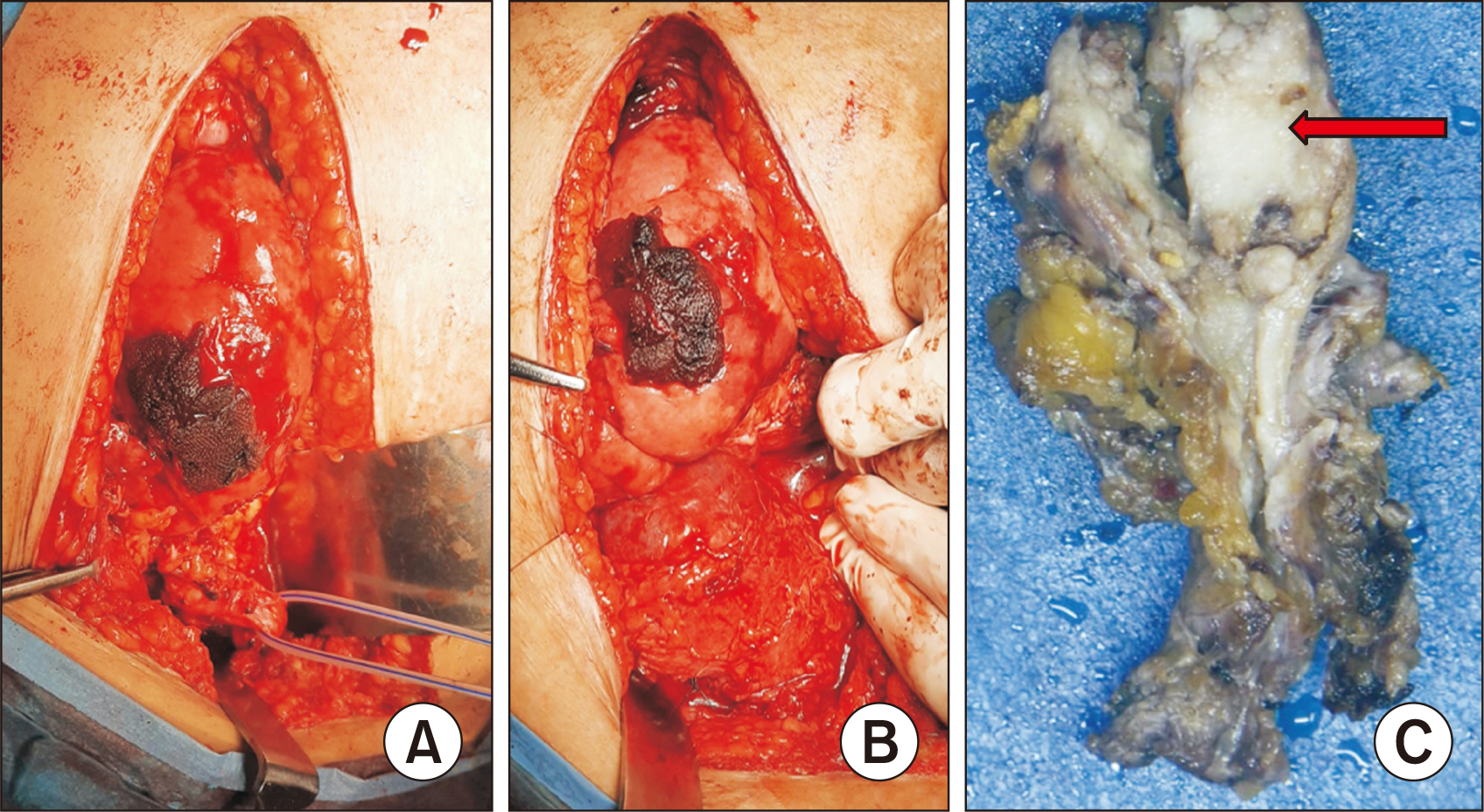
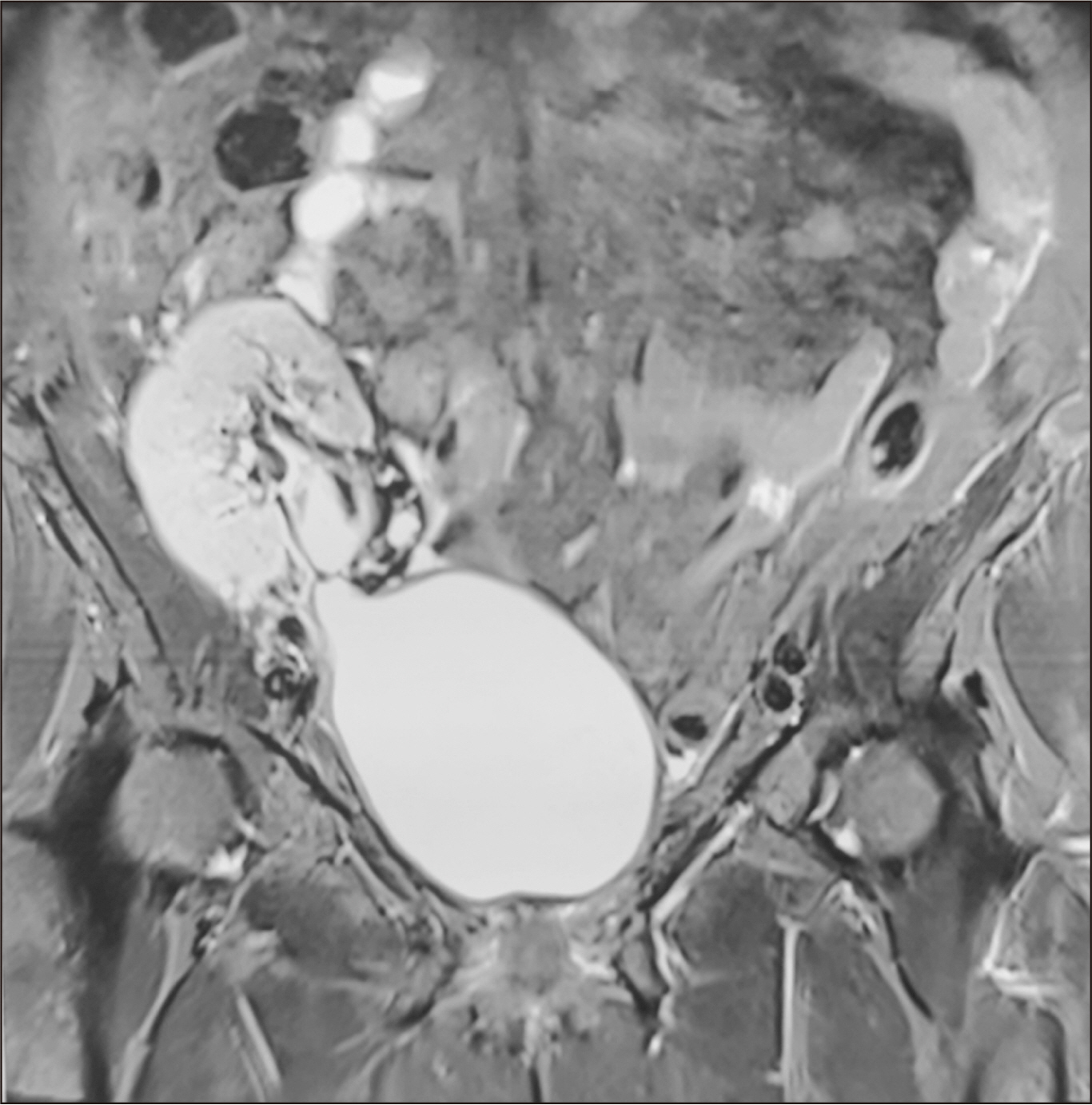
- Renal transplant recipients are prone to a high risk of subsequent upper tract urothelial carcinoma, occurring in both native and transplant ureters. We report a rare case of adenocarcinoma with yolk sac differentiation of the transplant ureter, which was managed successfully with transplant ureterectomy and pyelovesicostomy, thereby salvaging the functioning transplant kidney.
Keyword
Figure
Reference
-
1. Yu J, Lee CU, Kang M, Jeon HG, Jeong BC, Seo SI, et al. 2018; Incidences and oncological outcomes of urothelial carcinoma in kidney transplant recipients. Cancer Manag Res. 11:157–66. DOI: 10.2147/CMAR.S185796. PMID: 30636892. PMCID: PMC6307682.
Article2. Olsburgh J, Zakri RH, Horsfield C, Collins R, Fairweather J, O'Donnell P, et al. 2016; TCC in transplant ureter: when and when not to preserve the transplant kidney. Am J Transplant. 16:704–11. DOI: 10.1111/ajt.13533. PMID: 26731492.3. Sagnotta A, Dente M, Socciarelli F, Cacchi C, Stoppacciaro A, Balducci G. 2014; Primary adenocarcinoma of the renal pelvis: histologic features of a stepwise process from intestinal hyperplasia to dysplasia in a patient with chronic renal abscess. Int J Surg Pathol. 22:182–5. DOI: 10.1177/1066896913502225. PMID: 24008439.4. Hudson J, Arnason T, Merrimen JL, Lawen J. 2013; Intestinal type villous adenoma of the renal pelvis. Can Urol Assoc J. 7:E138–42. DOI: 10.5489/cuaj.257. PMID: 23671505. PMCID: PMC3650791.
Article5. Gullo CE, Fantin JP, Spessoto LC, Gatti M, Arruda JG, Antoniassi TD, et al. 2017; Renal cell carcinoma and mucinous adenocarcinoma of ureter after renal transplantation with neoplasm of urethra in follow up. Int J Sci. 6:77–80. DOI: 10.18483/ijSci.1202.6. Healy KA, Carney KJ, Osunkoya AO. 2010; Endometrioid adenocarcinoma in the native ureter of a renal transplant patient: case report and review of the literature. ScientificWorldJournal. 10:1714–22. DOI: 10.1100/tsw.2010.166. PMID: 20842317. PMCID: PMC5763830.
Article7. Chaudhary P, Agarwal R, Srinivasan S, Singh D. 2016; Primary adenocarcinoma of ureter: a rare histopathological variant. Urol Ann. 8:357–9. DOI: 10.4103/0974-7796.184885. PMID: 27453661. PMCID: PMC4944632.
Article8. Espejo-Herrera N, Condom-Mundó E. 2020; Yolk sac tumor differentiation in urothelial carcinoma of the urinary bladder: a case report and differential diagnosis. Diagn Pathol. 15:68. DOI: 10.1186/s13000-020-00983-3. PMID: 32493368. PMCID: PMC7271425.
Article9. Collins K, Alkashash AM, Hwang M, Kaimakliotis HZ, Cheng L, Idrees MT. 2022; Somatic-type yolk sac tumor arising as a predominant component of bladder urothelial carcinoma. Int J Surg Pathol. 30:207–13. DOI: 10.1177/10668969211030688. PMID: 34255554.
Article10. Bihari C, Rastogi A, Chandan KN, Yadav V, Panda D. 2013; Gastric adenocarcinoma with yolk sac tumor differentiation and liver metastasis of yolk sac tumor component. Case Rep Oncol Med. 2013:923596. DOI: 10.1155/2013/923596. PMID: 24294529. PMCID: PMC3835910.
Article11. Umeda H, Kikuchi S, Kuroda S, Yano S, Tanaka T, Noma K, et al. 2021; Long-term survival without recurrence after surgery for gastric yolk sac tumor-like carcinoma: a case report. Surg Case Rep. 7:111. DOI: 10.1186/s40792-021-01199-3. PMID: 33956241. PMCID: PMC8102656.
Article12. Ji M, Lu Y, Guo L, Feng F, Wan X, Xiang Y. 2013; Endometrial carcinoma with yolk sac tumor-like differentiation and elevated serum β-hCG: a case report and literature review. Onco Targets Ther. 6:1515–22. DOI: 10.2147/OTT.S51983. PMID: 24187502. PMCID: PMC3810345.13. Sookram J, Levin B, Barroeta J, Kenley K, Mehta P, Krill LS. 2019; A case of ovarian endometrioid adenocarcinoma with yolk sac differentiation and Lynch syndrome. Gynecol Oncol Rep. 27:60–4. DOI: 10.1016/j.gore.2019.01.001. PMID: 30723761. PMCID: PMC6348978.
Article14. Gutierrez-Dalmau A, Campistol JM. 2007; Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 67:1167–98. DOI: 10.2165/00003495-200767080-00006. PMID: 17521218.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Yolk Sac Tumor of the Cerebellar Hemisphere: Case Report
- A case of Primary Mediastinal Yolk Sac Tumor
- Yolk-sac Tumor of Testis in Male Infant with Pulmonary Metastasis: A Case Report
- Adenocarcinoma with Yolk Sac Tumor of the Stomach: Case Report with Review of the Literature and an Immunohistochemical Study
- A Case of Systemic Chemotherapy in Advanced Yolk Sac Carcinoma