Anat Cell Biol.
2023 Jun;56(2):219-227. 10.5115/acb.22.220.
Safflower seed oil, a rich source of linoleic acid, stimulates hypothalamic neurogenesis in vivo
- Affiliations
-
- 1Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
- 2Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
- 3PRASE and Biology Department, Faculty of Sciences-I, Lebanese University, Beirut, Lebanon
- KMID: 2544084
- DOI: http://doi.org/10.5115/acb.22.220
Abstract
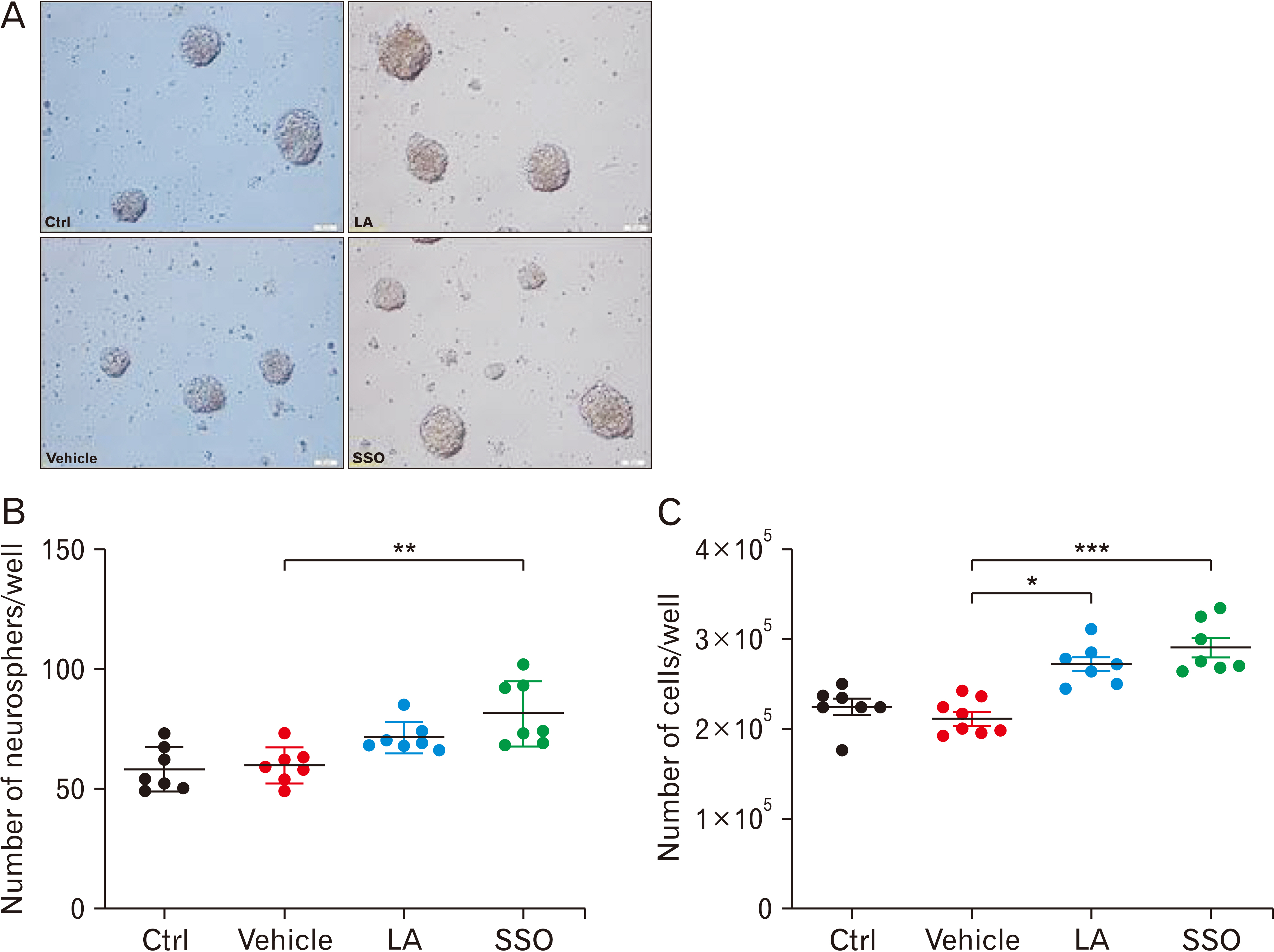
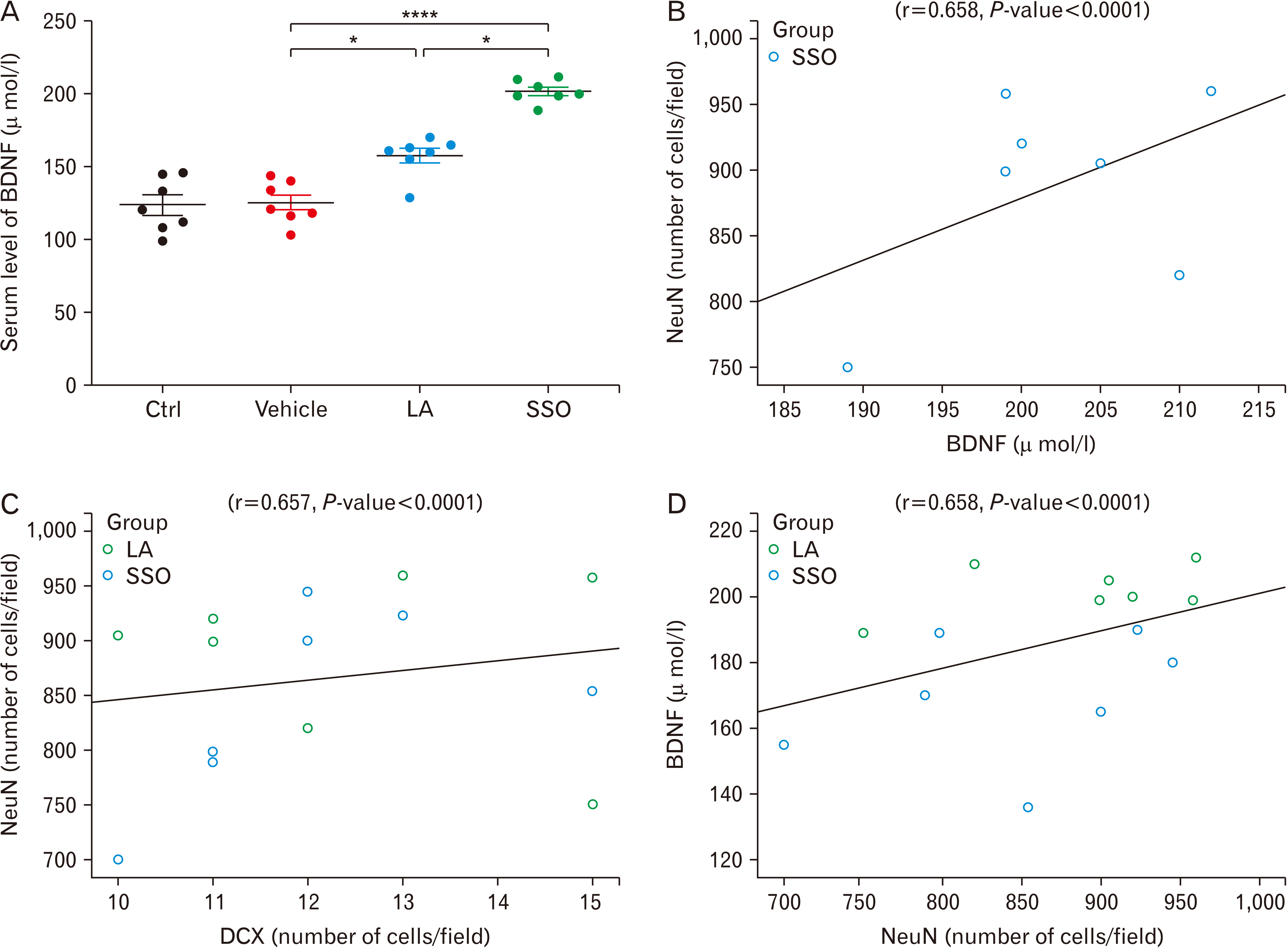
- Adult neurogenesis has been reported in the hypothalamus, subventricular zone and subgranular zone in the hippocamp. Recent studies indicated that new cells in the hypothalamus are affected by diet. We previously showed beneficial effects of safflower seed oil (SSO), a rich source of linoleic acid (LA; 74%), on proliferation and differentiation of neural stem cells (NSCs) in vitro. In this study, the effect of SSO on hypothalamic neurogenesis was investigated in vivo, in comparison to synthetic LA. Adult mice were treated with SSO (400 mg/kg) and pure synthetic LA (300 mg/kg), at similar concentrations of LA, for 8 weeks and then hypothalamic NSCs were cultured and subsequently used for Neurosphere-forming assay. In addition, serum levels of brain-derived neurotrophic factor (BNDF) were measured using enzyme-linked immunosorbent assay. Administration of SSO for 8 weeks in adult mice promoted the proliferation of NSCs isolated from SSO-treated mice. Immunofluorescence staining of the hypothalamus showed that the frequency of astrocytes (glial fibrillary acidic protein+ cells) are not affected by LA or SSO. However, the frequency of immature (doublecortin+ cells) and mature (neuronal nuclei+ cells) neurons significantly increased in LA- and SSO-treated mice, compared to vehicle. Furthermore, both LA and SSO caused a significant increase in the serum levels of BDNF. Importantly, SSO acted more potently than LA in all experiments. The presence of other fatty acids in SSO, such as oleic acid and palmitic acid, suggests that they could be responsible for SSO positive effect on hypothalamic proliferation and neurogenesis, compared to synthetic LA at similar concentrations.
Keyword
Figure
Reference
-
References
1. Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. 2004; Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 44:399–421. DOI: 10.1146/annurev.pharmtox.44.101802.121631. PMID: 14744252.
Article2. Ming GL, Song H. 2005; Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 28:223–50. DOI: 10.1146/annurev.neuro.28.051804.101459. PMID: 16022595.
Article3. Palmer TD, Ray J, Gage FH. 1995; FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 6:474–86. DOI: 10.1006/mcne.1995.1035. PMID: 8581317.
Article4. Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. 2008; PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 11:1392–401. DOI: 10.1038/nn.2220. PMID: 18849983. PMCID: PMC3842596.
Article5. Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G 2nd, Jiang L, Kang J, Nedergaard M, Goldman SA. 2003; Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 9:439–47. DOI: 10.1038/nm837. PMID: 12627226.
Article6. Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. 2010; Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 32:2042–52. DOI: 10.1111/j.1460-9568.2010.07521.x. PMID: 21143659.
Article7. Robins SC, Stewart I, McNay DE, Taylor V, Giachino C, Goetz M, Ninkovic J, Briancon N, Maratos-Flier E, Flier JS, Kokoeva MV, Placzek M. 2013; α-tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat Commun. 4:2049. DOI: 10.1038/ncomms3049. PMID: 23804023.
Article8. Eghlidospour M, Ghanbari A, Mortazavi SMJ, Azari H. 2017; Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem cells. Anat Cell Biol. 50:115–23. DOI: 10.5115/acb.2017.50.2.115. PMID: 28713615. PMCID: PMC5509895.
Article9. Pierce AA, Xu AW. 2010; De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J Neurosci. 30:723–30. DOI: 10.1523/JNEUROSCI.2479-09.2010. PMID: 20071537. PMCID: PMC3080014.10. Abbasnia K, Ghanbari A, Abedian M, Ghanbari A, Sharififar S, Azari H. 2015; The effects of repetitive transcranial magnetic stimulation on proliferation and differentiation of neural stem cells. Anat Cell Biol. 48:104–13. DOI: 10.5115/acb.2015.48.2.104. PMID: 26140221. PMCID: PMC4488638.
Article11. Poulose SM, Miller MG, Scott T, Shukitt-Hale B. 2017; Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr. 8:804–11. DOI: 10.3945/an.117.016261. PMID: 29141966. PMCID: PMC5683005.
Article12. Gordon N. 1997; Nutrition and cognitive function. Brain Dev. 19:165–70. DOI: 10.1016/S0387-7604(96)00560-8. PMID: 9134186.
Article13. Hamosh M, Salem N Jr. 1998; Long-chain polyunsaturated fatty acids. Biol Neonate. 74:106–20. DOI: 10.1159/000014017. PMID: 9691153.
Article14. Kelly GJ. 1984; Formation and fates of plant polyunsaturated fatty acids. Trends Biochem Sci. 9:502–3. DOI: 10.1016/0968-0004(84)90269-X.
Article15. Barnes PM, Bloom B, Nahin RL. 2008; Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. (12):1–23. DOI: 10.1037/e623942009-001. PMID: 19361005.
Article16. Ghareghani M, Zibara K, Azari H, Hejr H, Sadri F, Jannesar R, Ghalamfarsa G, Delaviz H, Nouri E, Ghanbari A. 2017; Safflower seed oil, containing oleic acid and palmitic acid, enhances the stemness of cultured embryonic neural stem cells through notch1 and induces neuronal differentiation. Front Neurosci. 11:446. DOI: 10.3389/fnins.2017.00446. PMID: 28824367. PMCID: PMC5540893. PMID: 66efc589a6fa45e19842f4d4ae55e563.
Article17. Sabzalian MR, Saeidi G, Mirlohi A. 2008; Oil content and fatty acid composition in seeds of three safflower species. J Am Oil Chem Soc. 85:717–21. DOI: 10.1007/s11746-008-1254-6.
Article18. Li J, Tang Y, Cai D. 2012; IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 14:999–1012. DOI: 10.1038/ncb2562. PMID: 22940906. PMCID: PMC3463771.
Article19. Calapai G. 2008; European legislation on herbal medicines: a look into the future. Drug Saf. 31:428–31. DOI: 10.2165/00002018-200831050-00009. PMID: 18422385.20. Kamelia E, Miko H, Karo MB, Hatta M. 2016; Neurogenesis and brain-derived neurotrophic factor levels in herbal therapy. Int J Res Med Sci. 4:4654–8. DOI: 10.18203/2320-6012.ijrms20163757.
Article21. Sakayori N, Osumi N. 2013; Polyunsaturated fatty acids and their metabolites in neural development and implications for psychiatric disorders. Curr Psychopharmacol. 2:73–83. DOI: 10.2174/2211556011302010073.
Article22. Park YS, Jang HJ, Lee KH, Hahn TR, Paik YS. 2006; Prolyl endopeptidase inhibitory activity of unsaturated fatty acids. J Agric Food Chem. 54:1238–42. DOI: 10.1021/jf052521h. PMID: 16478242.
Article23. Kim YJ, Nakatomi R, Akagi T, Hashikawa T, Takahashi R. 2005; Unsaturated fatty acids induce cytotoxic aggregate formation of amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutants. J Biol Chem. 280:21515–21. DOI: 10.1074/jbc.M502230200. PMID: 15799963.
Article24. Granholm AC, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K. 2008; Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 14:133–45. DOI: 10.3233/JAD-2008-14202. PMID: 18560126. PMCID: PMC2670571.
Article25. Okui T, Hashimoto M, Katakura M, Shido O. 2011; Cis-9,trans-11-conjugated linoleic acid promotes neuronal differentiation through regulation of Hes6 mRNA and cell cycle in cultured neural stem cells. Prostaglandins Leukot Essent Fatty Acids. 85:163–9. DOI: 10.1016/j.plefa.2011.06.001. PMID: 21723718.
Article26. Lledo PM, Alonso M, Grubb MS. 2006; Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 7:179–93. DOI: 10.1038/nrn1867. PMID: 16495940.
Article27. Recabal A, Caprile T, García-Robles MLA. 2017; Hypothalamic neurogenesis as an adaptive metabolic mechanism. Front Neurosci. 11:190. DOI: 10.3389/fnins.2017.00190. PMID: 28424582. PMCID: PMC5380718.
Article28. Pérez-Martín M, Cifuentes M, Grondona JM, López-Avalos MD, Gómez-Pinedo U, García-Verdugo JM, Fernández-Llebrez P. 2010; IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur J Neurosci. 31:1533–48. DOI: 10.1111/j.1460-9568.2010.07220.x. PMID: 20525067.
Article29. Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. 2001; Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 21:6706–17. DOI: 10.1523/JNEUROSCI.21-17-06706.2001. PMID: 11517260. PMCID: PMC6763082.
Article30. Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. 1998; Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 37:1553–61. DOI: 10.1016/S0028-3908(98)00141-5. PMID: 9886678.
Article31. Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. 2011; Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 14:399–404. DOI: 10.1017/S1461145710001008. PMID: 20860877.
Article32. Batailler M, Droguerre M, Baroncini M, Fontaine C, Prevot V, Migaud M. 2014; DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: a comparative study between mouse, sheep, and human tissues. J Comp Neurol. 522:1966–85. DOI: 10.1002/cne.23514. PMID: 24288185.
Article33. Ballout N, Frappé I, Péron S, Jaber M, Zibara K, Gaillard A. 2016; Development and maturation of embryonic cortical neurons grafted into the damaged adult motor cortex. Front Neural Circuits. 10:55. DOI: 10.3389/fncir.2016.00055. PMID: 27536221. PMCID: PMC4971105. PMID: 1e4b5c3689444f069c3ac3b07878ec42.
Article34. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. 2003; Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 467:1–10. DOI: 10.1002/cne.10874. PMID: 14574675.
Article35. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. 2005; Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 21:1–14. DOI: 10.1111/j.1460-9568.2004.03813.x. PMID: 15654838.
Article36. Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG Jr, Ozcan U. 2009; Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9:35–51. DOI: 10.1016/j.cmet.2008.12.004. PMID: 19117545.
Article37. Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA. 2009; High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 4:e5045. DOI: 10.1371/journal.pone.0005045. PMID: 19340313. PMCID: PMC2661137. PMID: a7d0e63a092644f5bc26ec40e48366f8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Arctii Fructus is a Prominent Dietary Source of Linoleic Acid for Reversing Epidermal Hyperproliferation of Guinea Pigs
- Effects of Safflower Seed Extracts and Bovine Bone on Regeneration of Bone Defects in Mongrel Dogs
- Fatty acid analysis and regulatory effects of citron (Citrus junos Sieb. ex TANAKA) seed oil on nitric oxide production, lipid accumulation, and leptin secretion
- Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments
- The influence of linoleic acid and ursolic acid on mouse peritoneal macrophage activity