J Korean Med Sci.
2023 Jun;38(25):e198. 10.3346/jkms.2023.38.e198.
Ethics Committees: Structure, Roles, and Issues
- Affiliations
-
- 1Department of Clinical Immunology and Rheumatology, King George’s Medical University, Lucknow, India
- 2Department of Clinical Rheumatology and Immunology, University Hospital in Krakow, Krakow, Poland
- 3National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland
- 4Department of Internal Medicine N2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
- 5Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, UK
- 6Department of Biology and Biochemistry, South Kazakhstan Medical Academy, Shymkent, Kazakhstan
- KMID: 2544011
- DOI: http://doi.org/10.3346/jkms.2023.38.e198
Abstract
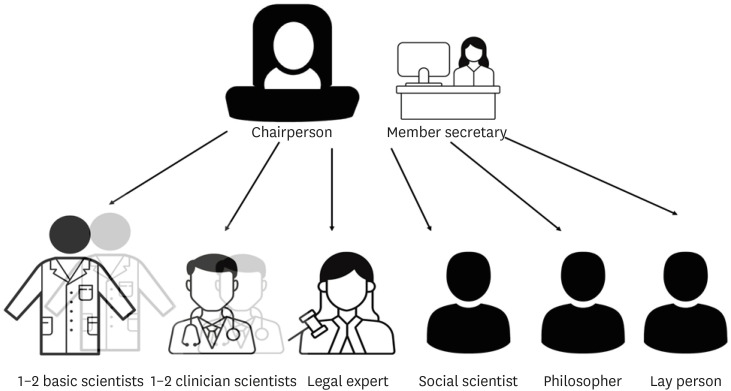
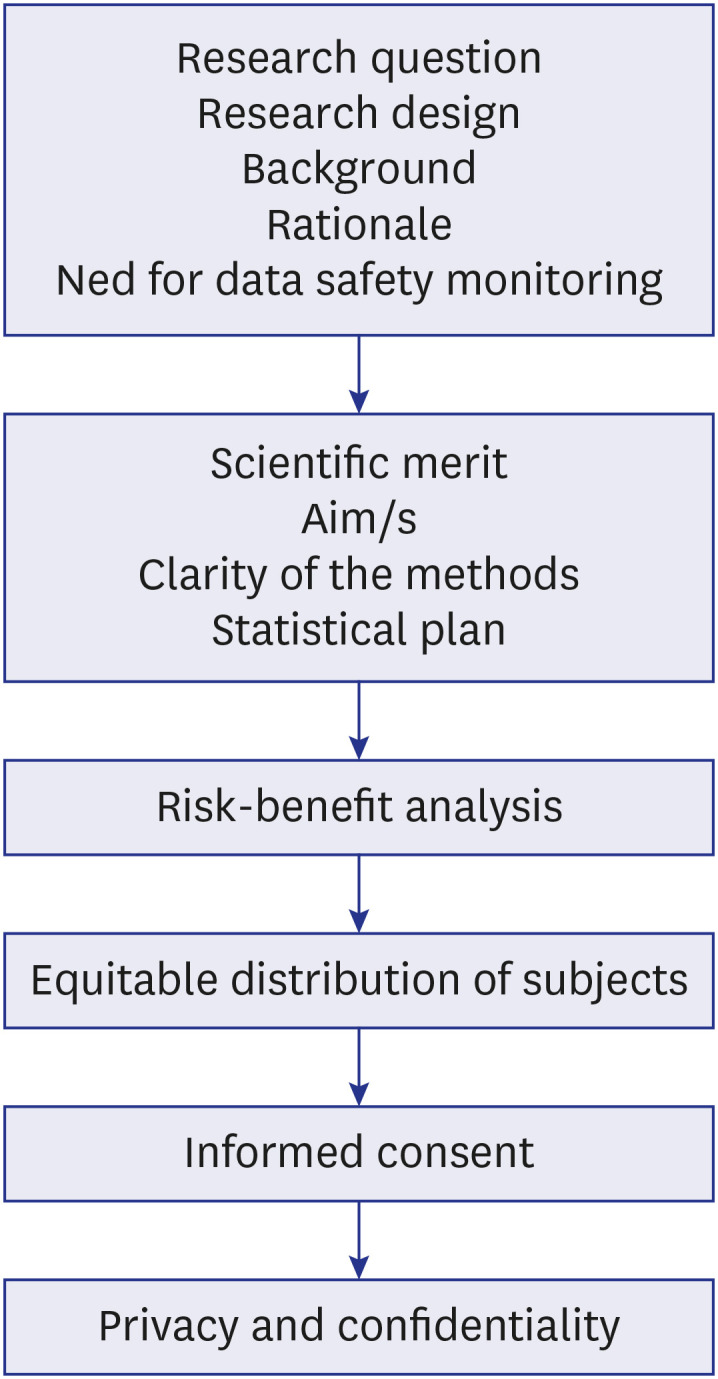
- An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty. MEDLINE, Scopus, and Directory of Open Access Journals were searched for studies relevant to this topic. This review is focused on the types of research articles that need EC approval, the submission process, and exemptions. It further highlights the constitution of ECs, their duties, the review process, and the assessment of the risk-benefit of the proposed research including privacy issues. It’s pertinent for academicians and researchers to abide by the rules and regulations put forth by ECs for upholding of human rights and protecting research subjects primarily, as well as avoiding other issues like retraction of publications. Despite various issues of cost, backlogs, lack of expertise, lesser representation of laypersons, need for multiple approvals for multisite projects, conflicts of interest, and monitoring of ongoing research for the continued safety of participants, the ECs form the central force in regulating research and participant safety. Data safety and monitoring boards complement the ECs for carrying out continuous monitoring for better protection of research subjects. The establishment of ECs has ensured safe study designs, the safety of human subjects along with the protection of researchers from before the initiation until the completion of a study.
Keyword
Figure
Cited by 1 articles
-
Research Integrity: Where We Are and Where We Are Heading
Alikhan Zhaksylyk, Olena Zimba, Marlen Yessirkepov, Burhan Fatih Kocyigit
J Korean Med Sci. 2023;38(47):e405. doi: 10.3346/jkms.2023.38.e405.
Reference
-
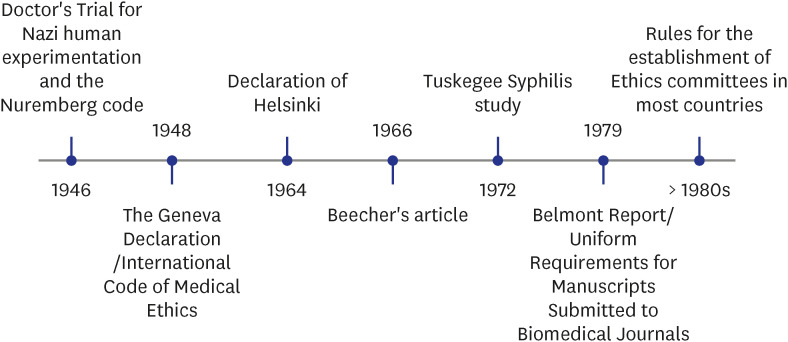
1. United States Holocaust Memorial Museum. The doctors trial: the medical case of the subsequent nuremberg proceedings. Updated 2023. Accessed March 17, 2023. https://encyclopedia.ushmm.org/content/en/article/the-doctors-trial-the-medical-case-of-the-subsequent-nuremberg-proceedings .2. Nuremberg Military Tribunal. The nuremberg code. JAMA. 1996; 276(20):1691. PMID: 11644854.3. Krugman S. The Willowbrook hepatitis studies revisited: ethical aspects. Rev Infect Dis. 1986; 8(1):157–162. PMID: 3952423.4. Beecher HK. Ethics and clinical research. N Engl J Med. 1966; 274(24):1354–1360. PMID: 5327352.5. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997; 277(11):925–926. PMID: 9062334.6. World Medical Association. WMA DOH 1964-2014. Updated 2023. Accessed March 17, 2023. https://www.wma.net/publications/wma-doh-1964-2014/ .7. National Center for Biotechnology Information. Ethics Committees. Updated 2023. Accessed April 5, 2023. https://www.ncbi.nlm.nih.gov/mesh/68017041 .8. Code of Federal Regulations. Part 46 - Protection of human subjects. Updated 2023. Accessed March 26, 2023. https://www.ecfr.gov/on/2018-07-19/title-45/subtitle-A/subchapter-A/part-46 .9. Office for Human Research Protections. Federal policy for the protection of human subjects (‘Common Rule’). Updated 2009. Accessed March 17, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/common-rule/index.html .10. Office for Human Research Protections. Read the Belmont report. Updated 2018. Accessed March 17, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html .11. Hennig W. Bioethics in China: although national guidelines are in place, their implementation remains difficult. EMBO Rep. 2006; 7(9):850–854. PMID: 16953195.12. Kumar NK. Bioethics activities in India. East Mediterr Health J. 2006; 12(Suppl 1):S56–S65. PMID: 17037690.13. Thatte UM, Marathe PA. Ethics Committees in India: past, present and future. Perspect Clin Res. 2017; 8(1):22–30. PMID: 28194334.14. Kim OJ, Park BJ, Sohn DR, Lee SM, Shin SG. Current status of the Institutional Review Boards in Korea: constitution, operation, and policy for protection of human research participants. J Korean Med Sci. 2003; 18(1):3–10. PMID: 12589079.15. Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 8th ed. Oxford, UK: Oxford University Press;2019.16. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle. 2019; 10(5):1143–1145. PMID: 31661195.17. Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011; 31(11):1409–1417. PMID: 21800117.18. Lapid MI, Clarke BL, Wright RS. Institutional Review Boards: what clinician researchers need to know. Mayo Clin Proc. 2019; 94(3):515–525. PMID: 30832791.19. Dutton JJ. Institutional Review Boards, Declaration of Helsinki, and HIPAA regulations. Ophthal Plast Reconstr Surg. 2013; 29(5):335–340.20. Wagner RM. Ethical review of research involving human subjects: when and why is IRB review necessary? Muscle Nerve. 2003; 28(1):27–39. PMID: 12811770.21. Byerly WG. Working with the Institutional Review Board. Am J Health Syst Pharm. 2009; 66(2):176–184. PMID: 19139484.22. World Health Organization (WHO) Centre for Health Development. WHO guidance on research methods for health emergency and disaster risk management. Updated 2021. Accessed March 30, 2023. https://extranet.who.int/kobe_centre/en/project-details/GUIDANCE_ResearchMethods_HealthEDRM .23. Orvis AK, Dellavalle RP. Institutional Review Board approval for surveys: why it is necessary. J Am Acad Dermatol. 2008; 59(4):718–719. PMID: 18793941.24. Gaur PS, Zimba O, Agarwal V, Gupta L. Reporting survey based studies - a primer for authors. J Korean Med Sci. 2020; 35(45):e398. PMID: 33230988.25. Bailey E, Mühlmann C, Rice S, Nedeljkovic M, Alvarez-Jimenez M, Sander L, et al. Ethical issues and practical barriers in internet-based suicide prevention research: a review and investigator survey. BMC Med Ethics. 2020; 21(1):37. PMID: 32404098.26. Balon R, Guerrero AP, Coverdale JH, Brenner AM, Louie AK, Beresin EV, et al. Institutional Review Board approval as an educational tool. Acad Psychiatry. 2019; 43(3):285–289. PMID: 30706434.27. Panda M, Heath GW, Desbiens NA, Moffitt B. Research status of case reports for medical school Institutional Review Boards. JAMA. 2007; 298(11):1277–1278. PMID: 17878419.28. Loe JD, Winkelman DA, Robertson CT. An assessment of the human subjects protection review process for exempt research. J Law Med Ethics. 2016; 44(3):481–491. PMID: 27587452.29. World Health Organization. Guidance for submissions of documents. Updated 2021. Accessed March 17, 2023. https://www.who.int/groups/research-ethics-review-committee/guidance-for-submissions-of-documents .30. European Medicines Agency. 3. Institutional Review Board/Independent Ethics Committee (IRB/IEC): ICH E6(R2) Good Clinical Practice. Updated 2018. Accessed March 26, 2023. https://ichgcp.net/3-institutional-review-boardindependent-ethics-committee-irbiec .31. Dal-Ré R. Waivers of informed consent in research with competent participants and the Declaration of Helsinki. Eur J Clin Pharmacol. 2023; 79(4):575–578. PMID: 36884061.32. Rebers S, Aaronson NK, van Leeuwen FE, Schmidt MK. Exceptions to the rule of informed consent for research with an intervention. BMC Med Ethics. 2016; 17(1):9. PMID: 26852412.33. Jones XM, Zimba O, Gupta L. Informed consent for scholarly articles during the COVID-19 pandemic. J Korean Med Sci. 2021; 36(3):e31. PMID: 33463097.34. Richards AD. Ethical guidelines for deliberately infecting volunteers with COVID-19. J Med Ethics. 2020; 46(8):502–504. PMID: 32461245.35. European Medicines Agency. ICH harmonised guideline integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2). Updated 2018. Accessed April 12, 2023. https://ichgcp.net/home. .36. Council for International Organizations of Medical Sciences. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva, Switzerland: Council for International Organizations of Medical Sciences;2002.37. Qiao H. A brief introduction to Institutional Review Boards in the United States. Pediatr Investig. 2018; 2(1):46–51.38. Hemminki E. Research Ethics Committees in the regulation of clinical research: comparison of Finland to England, Canada, and the United States. Health Res Policy Syst. 2016; 14(1):5. PMID: 26865158.39. Indian Council of Medical Research. Guidelines. Updated 2017. Accessed March 30, 2023. https://main.icmr.nic.in/guidelines?field_select_disease_tid=97 .40. Association for Accreditation of Human Research Protection Programs. The AAHRPP accreditation program. Updated 2019. Accessed March 20, 2023. http://www.aahrpp.org/resources/for-accreditation/procedure/procedure-doc-1/the-aahrpp-accreditation-program .41. Office for Human Research Protections. Regulations, policy & guidance. Updated 2020. Accessed March 20, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/index.html .42. Lidz CW, Simon LJ, Seligowski AV, Myers S, Gardner W, Candilis PJ, et al. The participation of community members on medical Institutional Review Boards. J Empir Res Hum Res Ethics. 2012; 7(1):1–6.43. National Institutes of Health. NIH policy for data and safety monitoring. Updated 1998. Accessed March 29, 2023. https://grants.nih.gov/grants/guide/notice-files/not98-084.html .44. Ellenberg SS, Fleming TR, DeMets DL. Data Monitoring Committees in Clinical Trials: A Practical Perspective. 2nd ed. Hoboken, NJ, USA: John Wiley & Sons, Inc.;2019.45. International Committee of Medical Journal Editors. Homepage of ICMJE. Updated 2023. Accessed March 26, 2023. https://www.icmje.org/ .46. Easter MM, Davis AM, Henderson GE. Confidentiality: more than a linkage file and a locked drawer. IRB. 2004; 26(2):13–17. PMID: 15069972.47. Regmi PR, Aryal N, Kurmi O, Pant PR, van Teijlingen E, Wasti SP. Informed consent in health research: challenges and barriers in low-and middle-income countries with specific reference to Nepal. Developing World Bioeth. 2017; 17(2):84–89.48. Center For Scientific Integrity, Inc.Retraction Watch Database. Updated 2023. Accessed March 29, 2023. http://retractiondatabase.org/RetractionSearch.aspx? .49. Moylan EC, Kowalczuk MK. Why articles are retracted: a retrospective cross-sectional study of retraction notices at BioMed Central. BMJ Open. 2016; 6(11):e012047.50. Campos-Varela I, Villaverde-Castañeda R, Ruano-Raviña A. Retraction of publications: a study of biomedical journals retracting publications based on impact factor and journal category. Gac Sanit. 2020; 34(5):430–434. PMID: 31530483.51. Kim YS, Ham DS. Analysis of consultations by the Committee for Publication Ethics of the Korean Association of Medical Journal Editors. Sci Ed. 2020; 7(2):184–188.52. Shetty YC, Singh KN, Marathe PA, Jalgaonkar SV, Gajbhiye S, Katkar J, et al. Reports of site monitoring visits by Institutional Ethics Committees in an Indian tertiary care hospital: a retrospective analysis. Indian J Med Ethics. 2019; 4(3):178–183. PMID: 31727613.53. Ochieng J, Ecuru J, Nakwagala F, Kutyabami P. Research site monitoring for compliance with ethics regulatory standards: review of experience from Uganda. BMC Med Ethics. 2013; 14(1):23. PMID: 23738971.54. Bain LE. Ethics approval: responsibilities of journal editors, authors and Research Ethics Committees. Pan Afr Med J. 2017; 28:200. PMID: 29610638.55. de Veras Santos EP, Guerriero IC. Professional and academic profile of the Brazilian Research Ethics Committees. BMC Med Ethics. 2022; 23(1):109. PMID: 36368994.56. Salim M, Hamid S. Independent Review of research proposals from ethical point of view in Pakistan. J Ayub Med Coll Abbottabad. 2018; 30(4):588–591. PMID: 30632343.57. Brahme R, Mehendale S. Profile and role of the members of Ethics Committees in hospitals and research organisations in Pune, India. Indian J Med Ethics. 2009; 6(2):78–84. PMID: 19517650.58. Varley PR, Feske U, Gao S, Stone RA, Zhang S, Monte R, et al. Time required to review research protocols at 10 veterans affairs Institutional Review Boards. J Surg Res. 2016; 204(2):481–489. PMID: 27565086.59. Silberman G, Kahn KL. Burdens on research imposed by Institutional Review Boards: the state of the evidence and its implications for regulatory reform. Milbank Q. 2011; 89(4):599–627. PMID: 22188349.60. Whitney SN, Schneider CE. Viewpoint: a method to estimate the cost in lives of ethics board review of biomedical research. J Intern Med. 2011; 269(4):396–402. PMID: 21410788.61. Sugarman J, Getz K, Speckman JL, Byrne MM, Gerson J, Emanuel EJ, et al. The cost of Institutional Review Boards in academic medical centers. N Engl J Med. 2005; 352(17):1825–1827. PMID: 15858200.62. Bramstedt KA, Kassimatis K. A study of warning letters issued to Institutional Review Boards by the United States Food and Drug Administration. Clin Invest Med. 2004; 27(6):316–323. PMID: 15675112.63. Kadam R, Karandikar S. Ethics Committees in India: facing the challenges! Perspect Clin Res. 2012; 3(2):50–56. PMID: 22701820.64. Zhou J. Problems and development strategies for Research Ethics Committees in China’s higher education institutions. J Med Ethics. 2020; medethics-2020-106768.65. Edwards SJ, Stone T, Swift T. Differences between Research Ethics Committees. Int J Technol Assess Health Care. 2007; 23(1):17–23. PMID: 17234012.66. Office for Human Research Protections. Revised Common Rule. Updated 2017. Accessed March 27, 2023. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/finalized-revisions-common-rule/index.html .67. Emanuel EJ, Wood A, Fleischman A, Bowen A, Getz KA, Grady C, et al. Oversight of human participants research: identifying problems to evaluate reform proposals. Ann Intern Med. 2004; 141(4):282–291. PMID: 15313744.68. Forster D. Independent Institutional Review Boards. Seton Hall Law Rev. 2003; 32(3):513–523. PMID: 12741410.69. Flynn KE, Hahn CL, Kramer JM, Check DK, Dombeck CB, Bang S, et al. Using central IRBs for multicenter clinical trials in the United States. PLoS One. 2013; 8(1):e54999. PMID: 23383026.70. National Cancer Institute (NCI) Central Institutional Review Board. Welcome to the CIRB. Updated 2023. Accessed March 30, 2023. https://ncicirb.org/ .71. Biomedical Research Alliance of New York. IRB services. Updated 2023. Accessed March 30, 2023. https://www.brany.com/irb-services/ .72. Borovečki A, ten Have H, Oresković S. Education of Ethics Committee members: experiences from Croatia. J Med Ethics. 2006; 32(3):138–142. PMID: 16507656.73. Davies H, Wells F, Druml C. How can we provide effective training for Research Ethics Committee members? A European assessment. J Med Ethics. 2008; 34(4):301–302. PMID: 18375685.74. Chaschin M, Mishatkina T, Guryleva M, Pustovit S, Zagirtdinova F, Sarymsakova B. Developing national systems for research ethics education in Eastern Europe and Central Asia. Pharmaceut Med. 2008; 22(5):289–295.75. Cooper JA, Borasky D, Rosenfeld S, Sugarman J. Challenges in the ethical review of research involving complementary and integrative medicine. Ther Innov Regul Sci. 2016; 50(3):337–341. PMID: 30227063.76. U.S. Food and Drug Administration. Establishment and operation of clinical trial data monitoring committees. Updated 2021. Accessed April 12, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishment-and-operation-clinical-trial-data-monitoring-committees .77. The Coronary Drug Project. Initial findings leading to modifications of its research protocol. JAMA. 1970; 214(7):1303–1313. PMID: 4320008.78. Lee BR, Lee KE. Independent data monitoring committees: review of current guidelines. Korean J Clin Pharm. 2016; 26(2):181–186.79. UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Operational Guidelines for the Establishment and Functioning of Data and Safety Monitoring Boards. Geneva, Switzerland: World Health Organization;2005.80. Sibbald B. Rofecoxib (Vioxx) voluntarily withdrawn from market. CMAJ. 2004; 171(9):1027–1028. PMID: 15505253.81. Federal Register. Pfizer, Inc.; Withdrawal of approval of familial adenomatous polyposis indication for CELEBREX. Updated 2012. Accessed March 30, 2023. https://www.federalregister.gov/documents/2012/06/08/2012-13900/pfizer-inc-withdrawal-of-approval-of-familial-adenomatous-polyposis-indication-for-celebrex .82. Coleman CH, Bouësseau MC. How do we know that Research Ethics Committees are really working? The neglected role of outcomes assessment in research ethics review. BMC Med Ethics. 2008; 9(1):6. PMID: 18373857.83. Van Toan N, Thi Hanh T. Retraction note: Improved treatment of asthma by using natural sources of antioxidants. Springerplus. 2014; 3(1):558. PMID: 25392122.84. Zhang JP, Zhang N, Chen X, Zhou Y, Jiang Z, Gao C, et al. Retraction note: Efficacy of dexmedetomidine as an adjunct to ropivacaine in bilateral dual-transversus abdominis plane blocks in patients with ovarian cancer who underwent cytoreductive surgery. BMC Anesthesiol. 2022; 22(1):188. PMID: 35715751.85. Retraction: Virtual reality-guided aortic valve leaflet reconstruction for type 0 bicuspid aortic stenosis. Interact Cardiovasc Thorac Surg. 2022; 35(6):ivac281. PMID: 36458985.86. Group BM. Retraction: Influence of maternal diet during lactation and use of formula feeds on development of atopic eczema in high risk infants. BMJ. 2015; 351:h5682. PMID: 26514599.87. Kantas T, Avendaño Capriles CA, Babor S, Tamdin T, Al-Rihani H, Thalla A, et al. Retraction: Relationship between chronic kidney disease staging and vitamin D deficiency: a retrospective study. Cureus. 2022; 14(3):r55. PMID: 35342661.88. Al Qteishat A, Kirov K, Bokov D. The profile of the key pro-inflammatory cytokines in the serum of patients with CD and their association with the disease severity and activity. BMC Gastroenterol. 2022; 22(1):477. PMID: 36404304.89. Retraction. Immunotherapy of HIV-infected patients with Gc protein-derived macrophage activating factor. J Med Virol. 2014; 86(11):1998. PMID: 25328930.90. Statement of Retraction: The association of interleukin-16 gene polymorphisms with IL-16 serum levels and risk of multiple sclerosis. Immunol Invest. 2023; 52(1):134–134. PMID: 33402009.91. El Refaeey AE, Abdelfattah H, Mosbah A, Gamal AM, Fayla E, Refaie W, et al. Retraction note: Is early intervention using Mansoura-VV uterine compression sutures an effective procedure in the management of primary atonic postpartum hemorrhage?: a prospective study. BMC Pregnancy Childbirth. 2023; 23(1):95. PMID: 36739375.92. Jayaweera JA, Reyes M, Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. 2019; 9(1):12637. PMID: 31477792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Some Future-oriented Roles and Phases for the Ethics Committee of KMA
- Enactment of Code of Medical Ethics, KMA and Its Application
- Bioethical Approach for Nursing Research -Focused on the Use of Research Ethics Committees
- Role and mission of the Committee of Health, Korean Medical Association
- Mission and Operation of Institutional Review Board