J Korean Acad Nurs.
2015 Jun;45(3):315-322. 10.4040/jkan.2015.45.3.315.
Bioethical Approach for Nursing Research -Focused on the Use of Research Ethics Committees
- Affiliations
-
- 1College of Nursing, Pusan National University, Yangsan, Korea. jeongis@pusan.ac.kr
- KMID: 2164372
- DOI: http://doi.org/10.4040/jkan.2015.45.3.315
Abstract
- PURPOSE
This paper was written to introduce methods of using the research ethics committee (RES) from requesting the initial review to reporting the close-out for nursing researchers.
METHODS
General ethical principles were described by reviewing the 'Bioethics and Safety Act' and other related guidelines, and constructing some questions and answers.
RESULTS
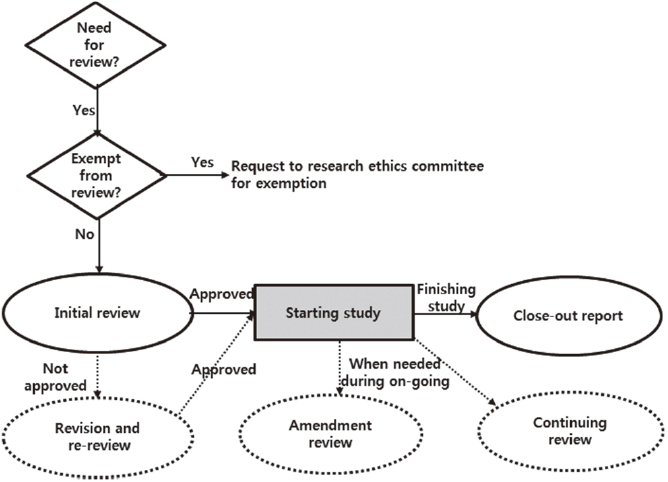
The results were composed of three parts; definition of RES, steps in using RES, and archiving. The 7 steps for using RES were; identifying whether the study needed to be reviewed, by the RES identifying whether the study could be exempted, requesting the initial review after preparing documents, requesting the re-review, requesting an amendment review, requesting a continuing review and reporting the close-out.
CONCLUSION
Nursing researchers need to receive RES approval before starting nursing research involving human subjects. Nursing researchers are urged to use the steps reported in this paper to receive RES approval easily and quickly.
Keyword
MeSH Terms
Figure
Reference
-
1. Park WK, Kim BI, Park HS, Lee IJ, Chung CS, Lee SD, et al. What is research ethics. Seoul: National Research Council for Economics, Humanities and Social Sciences;2014.2. Resnik DB. What is ethics in research & why is it important? [Internet]. Morrisville, NC: National Institute of Environmental Health Sciences;2011. cited 2015 May 1. Available from: http://www.niehs.nih.gov/research/resources/bioethics/whatis/.3. Shamoo A, Resnik DB. Responsible conduct of research. 2nd ed. New York, NY: Oxford University Press;2009.4. Korea Ministry of Government Legislation. Enforcement rule of pharmaceutical affairs act [Internet]. Sejong: Author;2014. cited 2015 May 1. Available from: http://www.law.go.kr/lsSc.do?-menuId=0&p1=&subMenu=1&nwYn=1§ion=&query=%EC%95%BD%EC%82%AC%EB%B2%95%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99&x=0&y=0#liBgcolor0.5. Korea Ministry of Government Legislation. Bioethics and safety act [Internet]. Sejong: Author;2014. cited 2015 March 1. Available from: http://www.law.go.kr/lsSc.do?menuId=0&p1=&subMenu=1&nwYn=1§ion=&query=%EC%83%9D%EB%AA%85%EC%9C%A4%EB%A6%AC+%EB%B0%8F+%EC%95%88%EC%A0%84&x=0&y=0#liBgcolor0.6. Korea Ministry of Government Legislation. Enforcement rule of bioethics and safety act [Internet]. Sejong: Author;2015. cited 2015 May 1. Available from: http://www.law.go.kr/lsSc.do?menuId=0&p1=&subMenu=1&nwYn=1§ion=&query=%EC%83%9D%EB%AA%85%EC%9C%A4%EB%A6%AC+%EB%B0%8F&x=37&y=9#AJAX.7. Korea Ministry of Government Legislation. Guideline for research ethics [Internet]. Busan: Pusan National University Research Institute of Nursing Science;2011. cited 2015 May 1. Available from: http://uwcms.pusan.ac.kr/user/boardList.action?command=view&siteId=708600&boardId=6293&boardSeq=219659.8. Kim OJ. Ethical handbook for investigators: Based on IRB, IACUC, IBC. Seoul: Seoul National University Hospital Biomedical Research Institute;2009.9. World Medical Association. WMA declaration of Helsinki: Ethical principles for medical research involving human subjects [Internet]. Ferney-Voltaire, FR: Author;2013. cited 2015 May 1. Available from: http://www.wma.net/en/30publications/10policies/b3/index.html.10. Polit DF, Beck CT. Nursing research: Generating and assessing evidence for nursing practice. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2012.11. Sugarman J, Mastroianni AC, Kahn JP, editors. Ethics of research with human subjects: Selected policies and resources. Frederick, MD: University Publishing Group;1998.12. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont report: Ethical principles and guidelines for the protection of human subjects of research [Internet]. Washington, DC: U.S. Department of Health, Education, and Welfare;1979. cited 2015 May 1. Available from: http://videocast.nih.gov/pdf/ohrp_appendix_belmont_report_vol_2.pdf.13. U.S. Department of Health & Human Services. Code of federal regulations: Title 45-Public welfare, part 46-Protection of human subjects [Internet]. Washington, DC: Author;2009. cited 2015 May 1. Available from: http://www.hhs.gov/ohrp/policy/ohrpregulations.pdf.14. Dunn CM, Chadwick GL. Protecting study volunteers in research. 3rd ed. Boston, MA: CenterWatch;2004.15. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3):189–198.16. Appelbaum PS, Grisso T. MacArthur competence assessment tool for clinical research (MacCAT-CR). Sarasota, FL: Professional Resource Press;2001.17. Ginsberg D, Gambrill S, Zisson S. Becoming a successful clinical research investigator. Boston, MA: CenterWatch;2009.18. Korea Ministry of Government Legislation. Enforcement decrees to personal information protection act [Internet]. Sejong: Author;2015. cited 2015 May 1. Available from: http://law.go.kr/lsSc.do?menuId=0&p1=&subMenu=1&nwYn=1§ion=&query=%EA%B0%9C%EC%9D%B8%EC%A0%95%EB%B3%B4&x=26&y=15#liBgcolor2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Institutional Review Boards and Bioethical Issues for Otologists and Audiologists
- Enactment of Code of Medical Ethics, KMA and Its Application
- Some Future-oriented Roles and Phases for the Ethics Committee of KMA
- Ethical issues in uterine transplantation
- Research Ethics in the Process of Conducting Research