Blood Res.
2023 Jun;58(2):91-98. 10.5045/br.2023.2022201.
Efficacy of plasmapheresis in neutropenic patients suffering from cytokine storm because of severe COVID-19 infection
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Isfahan, Iran
- 2Infectious Diseases and Tropical Medicine Research Center, Isfahan, Iran
- 3Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan, Iran
- 4Cancer Prevention Research Center Seyed Al-Shohada Hospital, Isfahan, Iran
- 5Mycology Reference Laboratory, Research Core Facilities Laboratory, Isfahan, Iran
- 6Acquired Immunodeficiency Research Center, Isfahan, Iran
- 7Department of Anesthesiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
- KMID: 2543949
- DOI: http://doi.org/10.5045/br.2023.2022201
Abstract
- Background
With the emergence of the coronavirus disease 2019 (COVID-19) and inability of healthcare systems to control the disease, various therapeutic theories with controversial responses have been proposed. Plasmapheresis was administered as a medication. However, the knowledge of its efficacy and indications is inadequate. This study evaluated the use of plasmapheresis in critically ill patients with cancer.
Methods
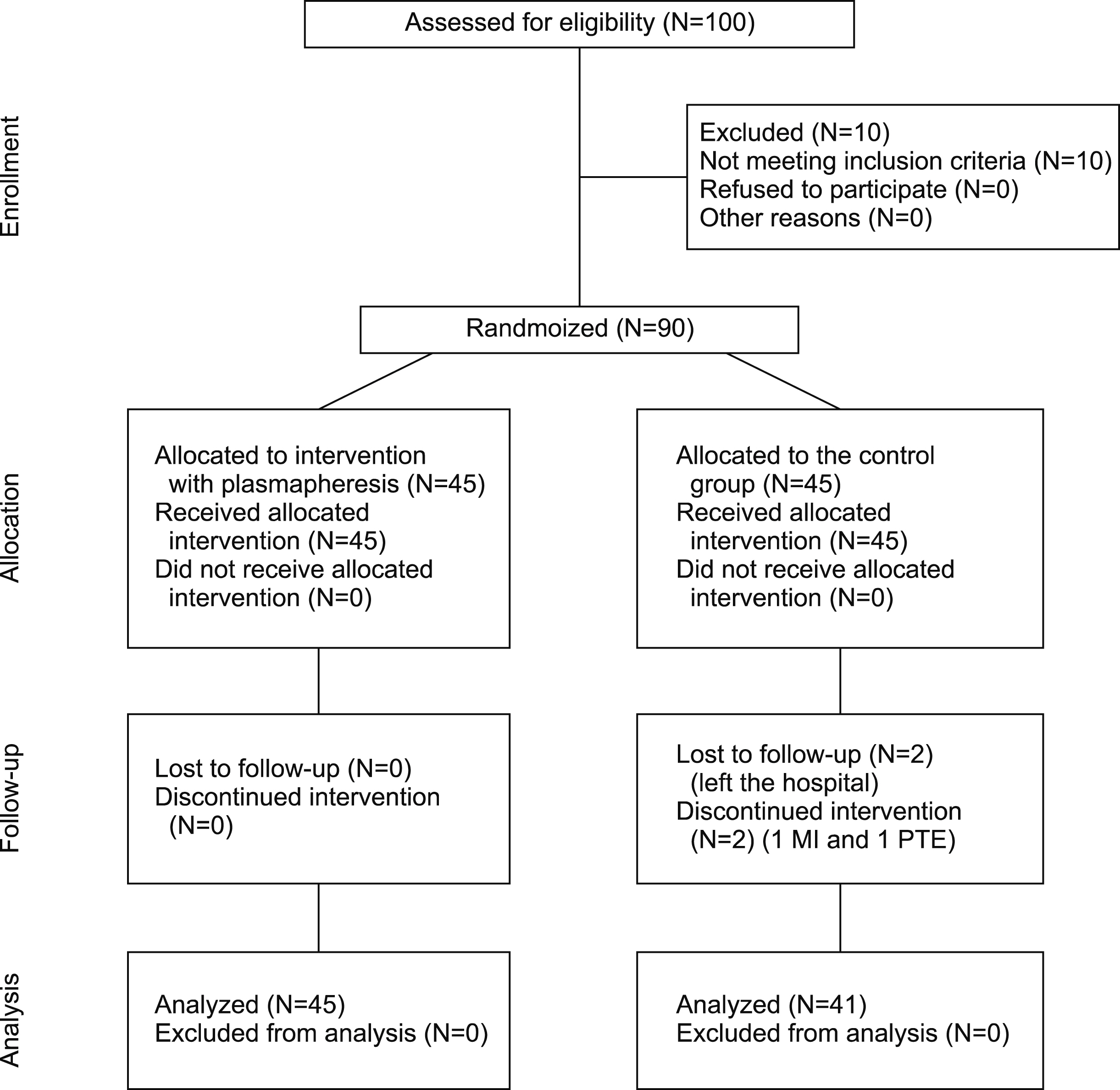
This randomized clinical trial was conducted on 86 patients with malignancies, including a control group (N=41) and an intervention group (N=45) with severe COVID-19 during 2020-21. Both groups were treated with routine medications for COVID-19 management according to national guidelines, and plasmapheresis was applied to the intervention group. C-reactive protein (CRP), D-dimer, ferritin, lactate dehydrogenase, hemoglobin, and white blood cell, polymorphonuclear, lymphocyte, and platelet levels were measured at admission and at the end of plasmapheresis. Other variables included neutrophil recovery, intensive care unit admission, intubation requirements, length of hospital stay, and hospitalization outcomes.
Results
CRP (P <0.001), D-dimer (P <0.001), ferritin (P =0.039), and hemoglobin (P =0.006) levels were significantly different between the groups after the intervention. Neutrophil recovery was remarkably higher in the case than in the control group (P <0.001). However, plasmapheresis did not affect the length of hospital stay (P =0.076), which could have significantly increased survival rates (P <0.001).
Conclusion
Based on the study findings, plasmapheresis led to a significant improvement in laboratory markers and survival rate in patients with severe COVID-19. These findings reinforce the value of plasmapheresis in cancer patients as a critical population suffering from neutropenia and insufficient immune responses.
Keyword
Figure
Reference
-
1. Harapan H, Itoh N, Yufika A, et al. 2020; Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 13:667–73. DOI: 10.1016/j.jiph.2020.03.019. PMID: 32340833. PMCID: PMC7142680.
Article2. Sadeghi S, Nasri P, Nasirian M, et al. 2022; On admission hemoglobin and albumin, as the two novel factors associated with thrombosis in COVID-19 pneumonia. J Renal Inj Prev. 11:9. DOI: 10.34172/jrip.2022.31957.
Article3. Wan Y, Shang J, Graham R, Baric RS, Li F. 2020; Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 94:e00127–20. DOI: 10.1128/JVI.00127-20. PMID: 31996437. PMCID: PMC7081895.
Article4. Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. 2020; Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 39:2085–94. DOI: 10.1007/s10067-020-05190-5. PMID: 32474885. PMCID: PMC7260446.
Article5. Sadeghi S, Nasirian M, Keivany E, Nasri P, Mirenayat MS. 2021; The demographic, clinical, and medical manifestations of pulmonary thromboembolism development in COVID-19. Blood Res. 56:293–300. DOI: 10.5045/br.2021.2021131. PMID: 34880142. PMCID: PMC8721446.
Article6. Jordan RE, Adab P, Cheng KK. 2020; Covid-19: risk factors for severe disease and death. BMJ. 368:m1198. DOI: 10.1136/bmj.m1198. PMID: 32217618.
Article7. Chen N, Zhou M, Dong X, et al. 2020; Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 395:507–13. DOI: 10.1016/S0140-6736(20)30211-7. PMID: 32007143.
Article8. Ye Q, Wang B, Mao J. 2020; The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 80:607–13. DOI: 10.1016/j.jinf.2020.03.037. PMID: 32283152. PMCID: PMC7194613.
Article9. Turgutkaya A, Yavaşoğlu İ, Bolaman Z. 2021; Application of plasmapheresis for Covid-19 patients. Ther Apher Dial. 25:248–9. DOI: 10.1111/1744-9987.13536. PMID: 32510799. PMCID: PMC7300871.
Article10. Hu B, Huang S, Yin L. 2021; The cytokine storm and COVID-19. J Med Virol. 93:250–6. DOI: 10.1002/jmv.26232. PMID: 32592501. PMCID: PMC7361342.
Article11. Sadeghi S, Keivany E, Nasirian M, Nasri P. 2021; On-admission versus in-hospital thromboembolism due to COVID-19 infection. What is the particular characteristic of those with early thrombotic events? Adv Respir Med. 89:484–92. DOI: 10.5603/ARM.a2021.0083. PMID: 34668181.
Article12. Balagholi S, Dabbaghi R, Eshghi P, Mousavi SA, Heshmati F, Mohammadi S. 2020; Potential of therapeutic plasmapheresis in treatment of COVID-19 patients: immunopathogenesis and coagulopathy. Transfus Apher Sci. 59:102993. DOI: 10.1016/j.transci.2020.102993. PMID: 33162341. PMCID: PMC7605792.
Article13. Schwartz J, Padmanabhan A, Aqui N, et al. 2016; Guidelines on the use of therapeutic apheresis in clinical practice-evidence‐based approach from the writing committee of the American Society for Apheresis: the seventh special issue. J Clin Apher. 31:149–62. DOI: 10.1002/jca.21470. PMID: 27322218.
Article14. Szczeklik W, Wawrzycka K, Włudarczyk A, et al. 2013; Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiol Intensive Ther. 45:7–13. DOI: 10.5603/AIT.2013.0002. PMID: 23572301.15. Tabibi S, Tabibi T, Conic RRZ, Banisaeed N, Streiff MB. 2020; Therapeutic plasma exchange: a potential management strategy for critically Ill COVID-19 patients. J Intensive Care Med. 35:827–35. DOI: 10.1177/0885066620940259. PMID: 32666875. PMCID: PMC7391476.
Article16. Kuriakose S, Singh K, Pau AK, et al. 2021; Developing treatment guidelines during a pandemic health crisis: lessons learned from COVID-19. Ann Intern Med. 174:1151–8. DOI: 10.7326/M21-1647. PMID: 34125574. PMCID: PMC8252833.
Article17. Marik P. 2020. EVMS critical care Covid-19 management protocol. Eastern Virginia Medical School;Norfolk, VA:18. Saeed GA, Gaba W, Shah A, et al. 2020; Correlation between chest CT severity scores and the clinical parameters of adult patients with COVID-19 pneumonia. Radiol Res Pract. 2021:6697677. DOI: 10.1101/2020.10.15.20213058.
Article19. Yang X, Yu Y, Xu J, et al. 2020; Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 8:475–81. DOI: 10.1016/S2213-2600(20)30079-5. PMID: 32105632. PMCID: PMC7102538.
Article20. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. 2020; Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 20:773. DOI: 10.1016/S1473-3099(20)30195-X. PMID: 32171390. PMCID: PMC7118515.
Article21. Wang Y, Zhang D, Du G, et al. 2020; Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 395:1569–78. DOI: 10.1016/S0140-6736(20)31022-9. PMID: 32423584. PMCID: PMC7190303.22. Molina JM, Delaugerre C, Le Goff J, et al. 2020; No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 50:384. DOI: 10.1016/j.medmal.2020.03.006. PMID: 32240719. PMCID: PMC7195369.
Article23. Rosca A, Balcaen T, Lanoix JP, et al. 2022; Mortality risk and antibiotic use for COVID-19 in hospitalized patients over 80. Biomed Pharmacother. 146:112481. DOI: 10.1016/j.biopha.2021.112481. PMID: 35062049. PMCID: PMC8712262.
Article24. Hassaniazad M, Vahedi MS, Samimagham HR, et al. 2021; Improvement of clinical outcome, laboratory findings and inflammatory cytokines levels using plasmapheresis therapy in severe COVID-19 cases. Respir Med. 189:106669. DOI: 10.1016/j.rmed.2021.106669. PMID: 34757278. PMCID: PMC8547850.
Article25. Adeli SH, Asghari A, Tabarraii R, et al. 2020; Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol Arch Intern Med. 130:455–8. DOI: 10.20452/pamw.15340. PMID: 32380821.26. Dogan L, Kaya D, Sarikaya T, et al. 2020; Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. 87:155–8. DOI: 10.1016/j.bbi.2020.05.022. PMID: 32389697. PMCID: PMC7204750.
Article27. Morath C, Weigand MA, Zeier M, Speer C, Tiwari-Heckler S, Merle U. 2020; Plasma exchange in critically ill COVID-19 patients. Crit Care. 24:481. DOI: 10.1186/s13054-020-03171-3. PMID: 32753056. PMCID: PMC7399583. PMID: b957fa8daf5e4ae2afef00e19f24f9ce.
Article28. Rimmer E, Houston BL, Kumar A, et al. 2014; The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 18:699. DOI: 10.1186/s13054-014-0699-2. PMID: 25527094. PMCID: PMC4318234.
Article29. Yang XH, Sun RH, Zhao MY, et al. 2020; Expert recommendations on blood purification treatment protocol for patients with severe COVID-19. Chronic Dis Transl Med. 6:106–14. DOI: 10.1016/j.cdtm.2020.04.002. PMID: 32346492. PMCID: PMC7186198.30. Fonseka CL, Lekamwasam S. 2018; Role of plasmapheresis and extracorporeal membrane oxygenation in the treatment of leptospirosis complicated with pulmonary hemorrhages. J Trop Med. 2018:4520185. DOI: 10.1155/2018/4520185. PMID: 30631369. PMCID: PMC6304550. PMID: f5907cdc42ee417db54a5582a8f9dd0d.
Article31. Schwindenhammer V, Girardot T, Chaulier K, et al. 2019; oXiris® use in septic shock: experience of two French centres. Blood Purif. 47(Suppl 3):1–7. DOI: 10.1159/000499510. PMID: 30982028.
Article32. Bucciarelli S, Espinosa G, Cervera R, et al. 2006; Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 54:2568–76. DOI: 10.1002/art.22018. PMID: 16868979.
Article33. Novacescu AN, Duma G, Buzzi B, et al. 2022; Therapeutic plasma exchange followed by convalescent plasma transfusion in severe and critically ill COVID‑19 patients: a single centre non-randomized controlled trial. Exp Ther Med. 23:76. DOI: 10.3892/etm.2021.10999. PMID: 34934447. PMCID: PMC8652389.
Article34. Donnelly SC, Strieter RM, Kunkel SL, et al. 1993; Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 341:643–7. DOI: 10.1016/0140-6736(93)90416-E. PMID: 8095568.
Article35. Wang R, Pan W, Jin L, et al. 2020; Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 471:88–102. DOI: 10.1016/j.canlet.2019.11.039. PMID: 31812696.
Article36. Hashemian SM, Mortaz E, Tabarsi P, et al. 2014; Elevated CXCL-8 expression in bronchoalveolar lavage correlates with disease severity in patients with acute respiratory distress syndrome resulting from tuberculosis. J Inflamm (Lond). 11:21. DOI: 10.1186/1476-9255-11-21. PMID: 25110464. PMCID: PMC4126912.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reperfusion injury or cytokine storm? Utilizing plasmapheresis in severe trauma-induced multiorgan failure: a case report
- Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Therapy, Preclinical and Clinical Evidence
- A systemic study on the vulnerability and fatality of prostate cancer patients towards COVID-19 through analysis of the TMPRSS2, CXCL10 and their co-expressed genes
- Effectiveness of Mavrilimumab in Viral Infections Including SARS-CoV-2 Infection - A Brief Review
- COVID-19 and Cancer: Questions to Be Answered