Int J Thyroidol.
2023 May;16(1):128-133. 10.11106/ijt.2023.16.1.128.
Ultrasonographic Development and Progression of a Thyroid Nodule in a Girl with TPO-Mutated Dyshormonogenesis during Levothyroxine Supplementation
- Affiliations
-
- 1Department of Pediatrics and Adolescent Medicine, Chungbuk National University Hospital, Cheongju, Korea
- 2Department of Pediatrics, Chungbuk National University College of Medicine, Cheongju, Korea
- KMID: 2543021
- DOI: http://doi.org/10.11106/ijt.2023.16.1.128
Abstract
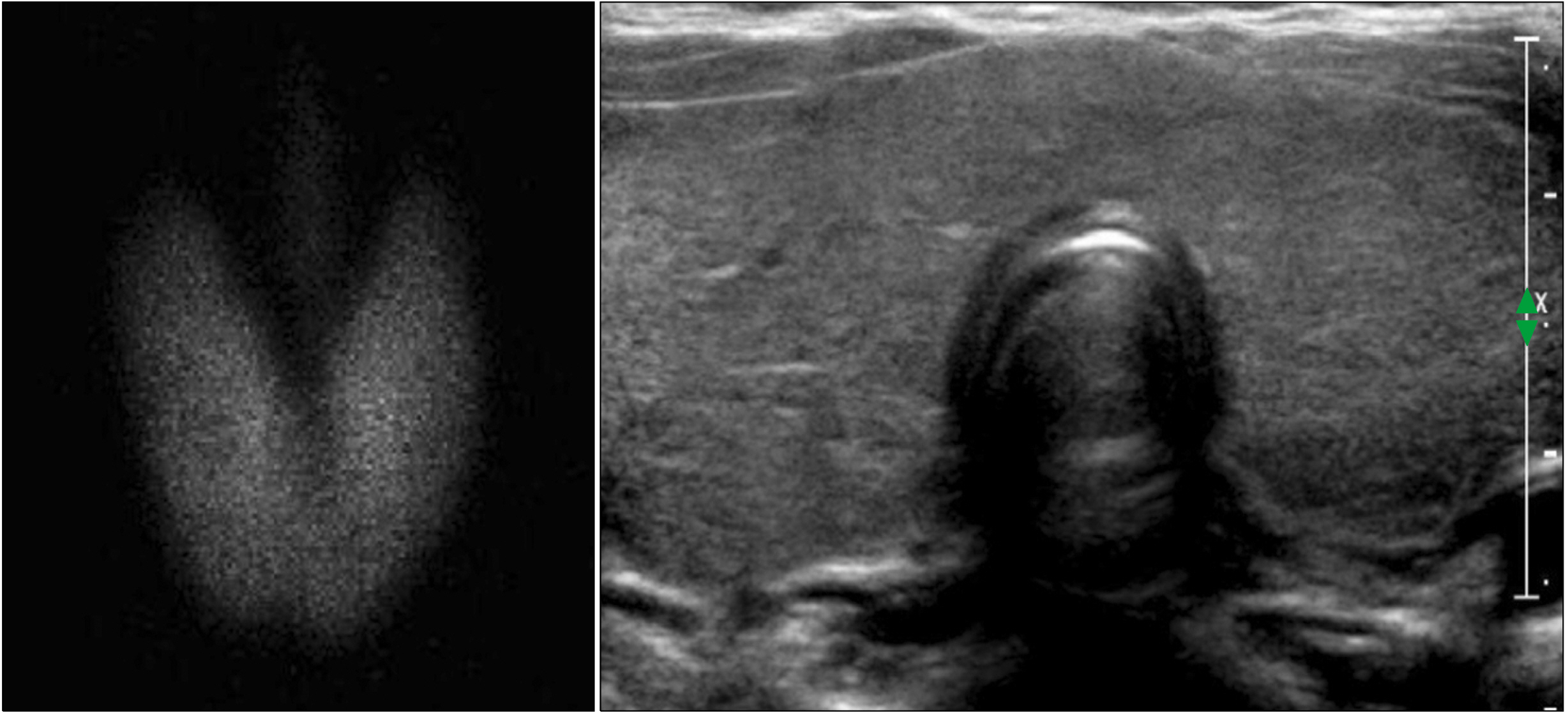
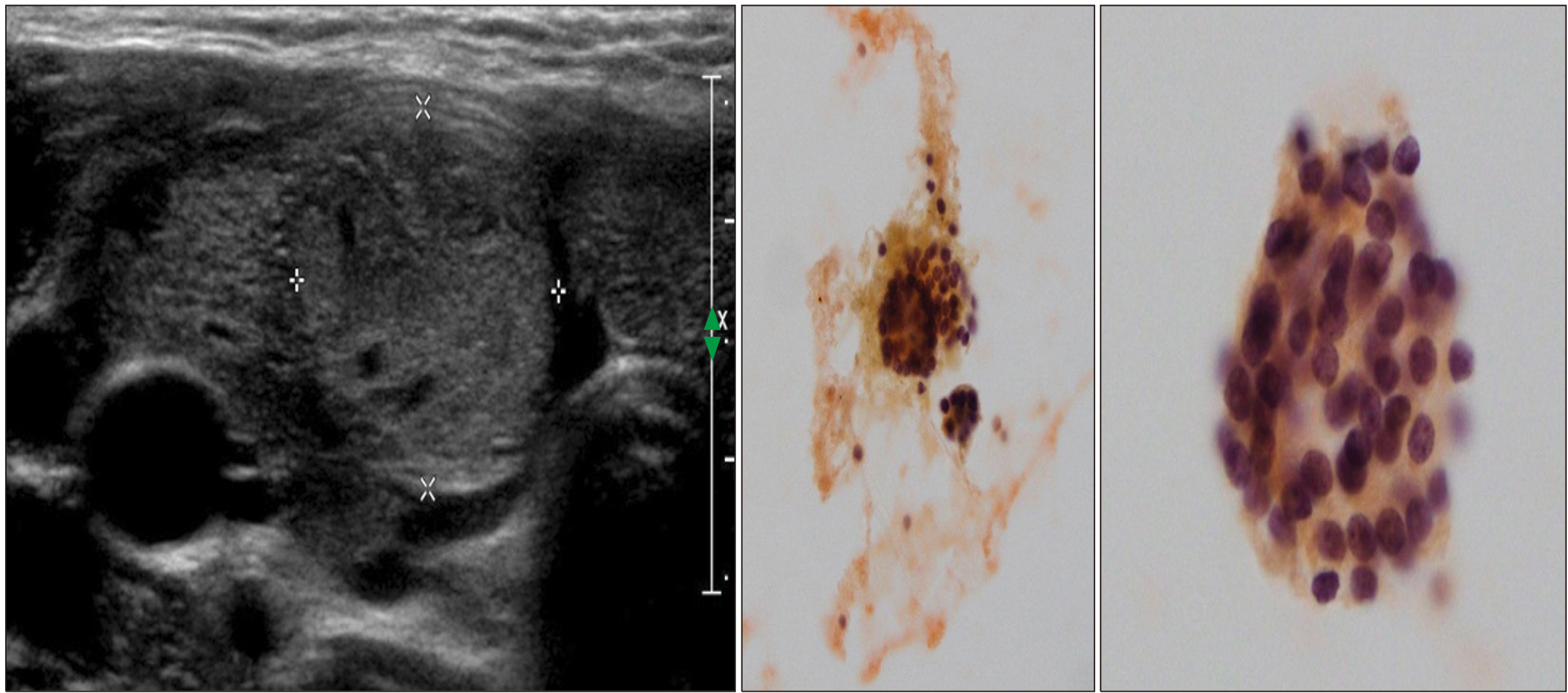
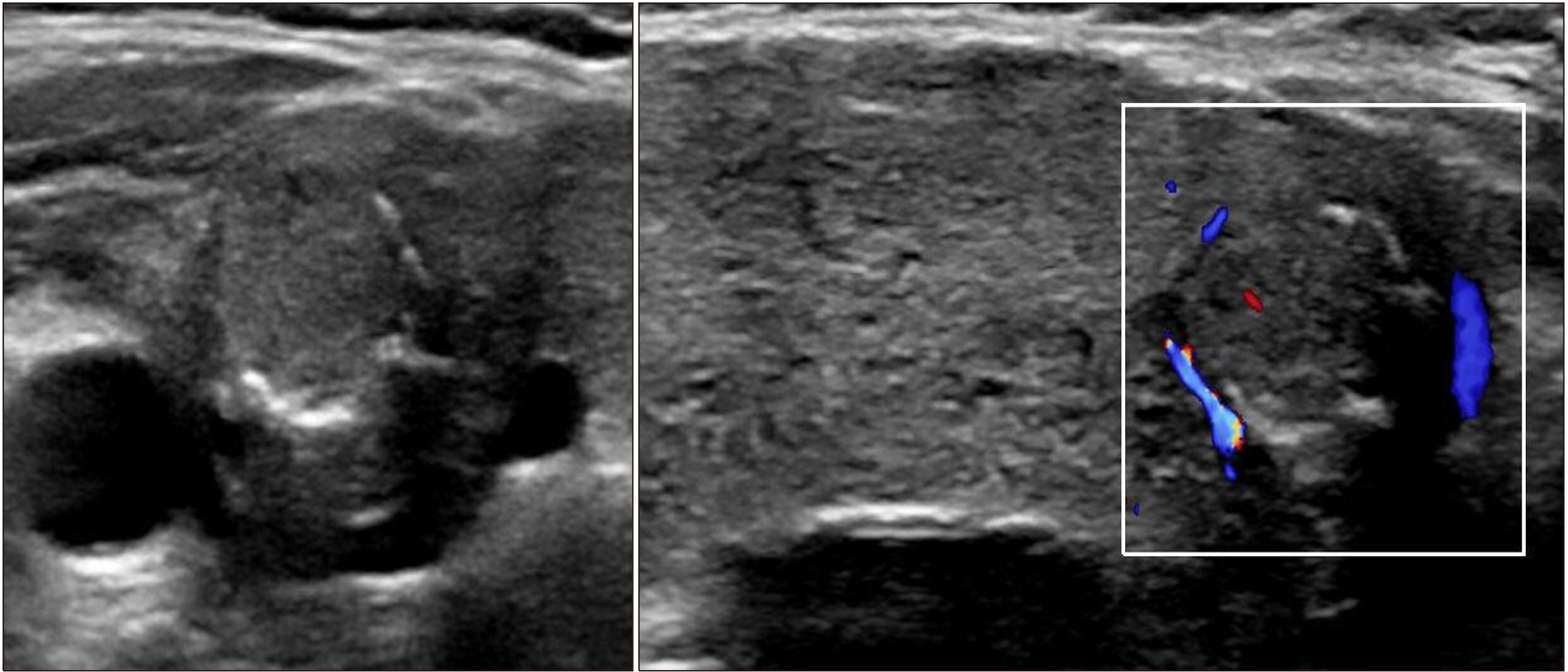
- Dyshormonogenesis is caused by genetic defects in thyroid hormone synthesis. The most common form is thyroid peroxidase (TPO) deficiency. Clinically variable degree of hypothyroidism and thyroid gland enlargement depend on the severity of the defect. We report 22-year-old female with congenital hypothyroidism (CH) caused by TPO deficiency. Since goitrous CH was diagnosed at 8-year-old, L-thyroxine has been supplemented. Her goiter size was fluctuated according to the compliance on the medication. After 3.5 years of medication, ultrasonography found solid nodule, which was interpreted as nodular hyperplasia pathologically. The nodule size did not change during recent 10 years except peripheral calcification. Genetic analysis using NGS for CH revealed compound heterozygous variants of c.2757del;p.(Met921Trpfs*53) and c.1580G>T;p.(Trp527Leu) in TPO gene. The first variant inherited from asymptomatic mother is pathogenic frame-shift mutation associated with stop codon, and the second one inherited from her asymptomatic father is predicted as deleterious in bioinformatics software program. From this case, we have observed that nodular change and calcification developed from diffuse enlarged goiter in dyshormonogenetic patient. Early molecular diagnosis of dyshormonogenesis and TSH suppression is important for not developing thyroid nodules in case of childhood euthyroid goiter without thyroid autoantibodies.
Figure
Reference
-
References
1. Grasberger H, Refetoff S. 2011; Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr. 23(4):421–8. DOI: 10.1097/MOP.0b013e32834726a4. PMID: 21543982. PMCID: PMC3263319.
Article2. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. 2014; European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 99(2):363–84. DOI: 10.1210/jc.2013-1891.
Article3. Kumar PG, Anand SS, Sood V, Kotwal N. 2005; Thyroid dyshormonogenesis. Indian Pediatr. 42(12):1233–5.4. Sriphrapradang C, Thewjitcharoen Y, Chanprasertyothin S, Nakasatien S, Himathongkam T, Trachoo O. 2016; A novel mutation in thyroid peroxidase gene causing congenital goitrous hypothyroidism in a German-Thai patient. J Clin Res Pediatr Endocrinol. 8(2):241–5. DOI: 10.4274/jcrpe.2503. PMID: 26761947. PMCID: PMC5096484.5. Kwak MJ. 2018; Clinical genetics of defects in thyroid hormone synthesis. Ann Pediatr Endocrinol Metab. 23(4):169–75. DOI: 10.6065/apem.2018.23.4.169. PMID: 30599477. PMCID: PMC6312914.
Article6. Abramowicz MJ, Targovnik HM, Varela V, Cochaux P, Krawiec L, Pisarev MA, et al. 1992; Identification of a mutation in the coding sequence of the human thyroid peroxidase gene causing congenital goiter. J Clin Invest. 90(4):1200–4. DOI: 10.1172/JCI115981. PMID: 1401057. PMCID: PMC443160.
Article7. Sun F, Zhang JX, Yang CY, Gao GQ, Zhu WB, Han B, et al. 2018; The genetic characteristics of congenital hypothyroidism in China by comprehensive screening of 21 candidate genes. Eur J Endocrinol. 178(6):623–33. DOI: 10.1530/EJE-17-1017. PMID: 29650690. PMCID: PMC5958289.8. Sorapipatcharoen K, Tim-Aroon T, Mahachoklertwattana P, Chantratita W, Iemwimangsa N, Sensorn I, et al. 2020; DUOX2 variants are a frequent cause of congenital primary hypothyroidism in Thai patients. Endocr Connect. 9(11):1121–34. DOI: 10.1530/EC-20-0411. PMID: 33310921. PMCID: PMC7774760.
Article9. Park KJ, Park HK, Kim YJ, Lee KR, Park JH, Park JH, et al. 2016; DUOX2 mutations are frequently associated with congenital hypothyroidism in the Korean population. Ann Lab Med. 36(2):145–53. DOI: 10.3343/alm.2016.36.2.145. PMID: 26709262. PMCID: PMC4713848.
Article10. Ghossein RA, Rosai J, Heffess C. 1997; Dyshormonogenetic goiter: a clinicopathologic study of 56 cases. Endocr Pathol. 8(4):283–92. DOI: 10.1007/BF02739930. PMID: 12114789.
Article11. Avbelj M, Tahirovic H, Debeljak M, Kusekova M, Toromanovic A, Krzisnik C, et al. 2007; High prevalence of thyroid peroxidase gene mutations in patients with thyroid dyshormonogenesis. Eur J Endocrinol. 156(5):511–9. DOI: 10.1530/EJE-07-0037. PMID: 17468186.
Article12. Corrias A, Mussa A. 2013; Thyroid nodules in pediatrics: which ones can be left alone, which ones must be investigated, when and how. J Clin Res Pediatr Endocrinol. 5 Suppl 1(Suppl 1):57–69.13. Chertok Shacham E, Ishay A, Irit E, Pohlenz J, Tenenbaum-Rakover Y. 2012; Minimally invasive follicular thyroid carcinoma developed in dyshormonogenetic multinodular goiter due to thyroid peroxidase gene mutation. Thyroid. 22(5):542–6. DOI: 10.1089/thy.2011.0478.
Article14. Tobias L, Elias-Assad G, Khayat M, Admoni O, Almashanu S, Tenenbaum-Rakover Y. 2021; Long-term outcome of patients with TPO mutations. J Clin Med. 10(17):3898. DOI: 10.3390/jcm10173898. PMID: 34501348. PMCID: PMC8432017.
Article15. Raef H, Al-Rijjal R, Al-Shehri S, Zou M, Al-Mana H, Baitei EY, et al. 2010; Biallelic p.R2223H mutation in the thyroglobulin gene causes thyroglobulin retention and severe hypothyroidism with subsequent development of thyroid carcinoma. J Clin Endocrinol Metab. 95(3):1000–6. DOI: 10.1210/jc.2009-1823. PMID: 20089614.16. Sparling DP, Fabian K, Harik L, Jobanputra V, Anyane- Yeboa K, Oberfield SE, et al. 2016; Congenital hypothyroidism and thyroid dyshormonogenesis: a case report of siblings with a newly identified mutation in thyroperoxidase. J Pediatr Endocrinol Metab. 29(5):627–31. DOI: 10.1515/jpem-2015-0253. PMID: 26894573. PMCID: PMC4853235.
Article17. Szanto I, Pusztaszeri M, Mavromati M. 2019; H(2)O(2) metabolism in normal thyroid cells and in thyroid tumorigenesis: focus on NADPH oxidases. Antioxidants (Basel). 8(5):126. DOI: 10.3390/antiox8050126. PMID: 31083324. PMCID: PMC6563055.18. Penna G, Rubio IGS, Brust ES, Cazarin J, Hecht F, Alkmim NR, et al. 2021; Congenital hypothyroidism and thyroid cancer. Endocr Relat Cancer. 28(9):R217–R30. DOI: 10.1530/ERC-21-0159. PMID: 34378152.19. Richman DM, Benson CB, Doubilet PM, Peters HE, Huang SA, Asch E, et al. 2018; Thyroid nodules in pediatric patients: sonographic characteristics and likelihood of cancer. Radiology. 288(2):591–9. DOI: 10.1148/radiol.2018171170. PMID: 29714678. PMCID: PMC6369930.20. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ. et. 2016; al. 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancers. Int J Thyroidol. 9:59–126. DOI: 10.11106/ijt.2016.9.2.59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical genetics of defects in thyroid hormone synthesis
- Genetic Variations of Congenital Hypothyroidism
- The Significance of Immunohistochemical Staining in Thyroid Nodule: TPO and Galectin-3
- The comparisons in ultrasonographic evaluation with radioisotope thyroid scanning in thyroid nodule
- Autoimmune Thyroid Diseases