Ann Pediatr Endocrinol Metab.
2018 Dec;23(4):169-175. 10.6065/apem.2018.23.4.169.
Clinical genetics of defects in thyroid hormone synthesis
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea. glorymj0123@gmail.com
- KMID: 2432279
- DOI: http://doi.org/10.6065/apem.2018.23.4.169
Abstract
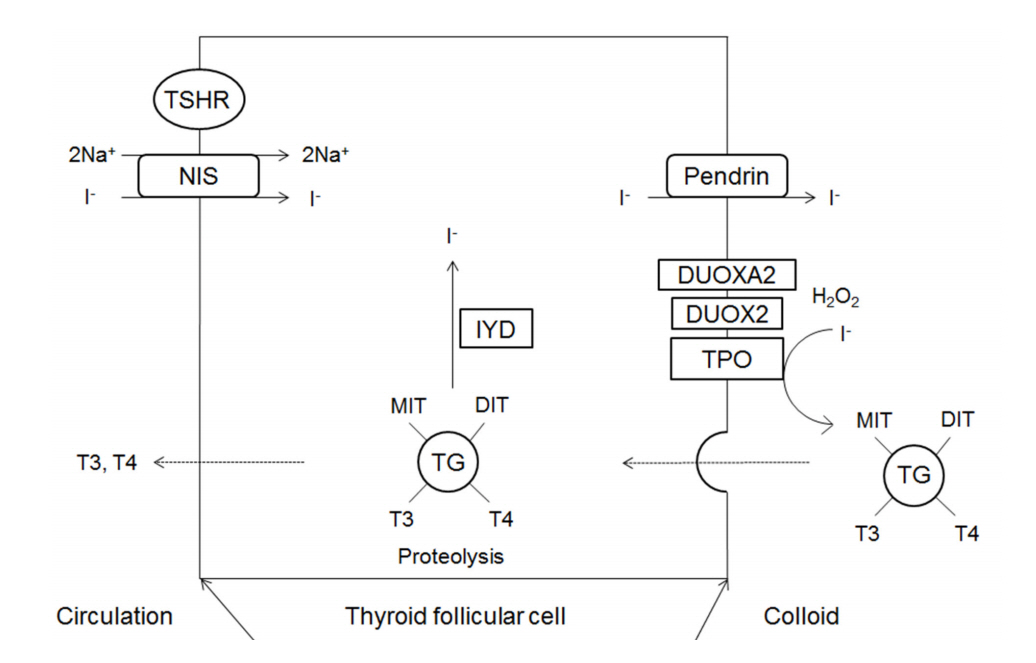
- Thyroid dyshormonogenesis is characterized by impairment in one of the several stages of thyroid hormone synthesis and accounts for 10%-15% of congenital hypothyroidism (CH). Seven genes are known to be associated with thyroid dyshormonogenesis: SLC5A5 (NIS), SCL26A4 (PDS), TG, TPO, DUOX2, DUOXA2, and IYD (DHEAL1). Depending on the underlying mechanism, CH can be permanent or transient. Inheritance is usually autosomal recessive, but there are also cases of autosomal dominant inheritance. In this review, we describe the molecular basis, clinical presentation, and genetic diagnosis of CH due to thyroid dyshormonogenesis, with an emphasis on the benefits of targeted exome sequencing as an updated diagnostic approach.
Figure
Cited by 1 articles
-
Ultrasonographic Development and Progression of a Thyroid Nodule in a Girl with
TPO -Mutated Dyshormonogenesis during Levothyroxine Supplementation
Jisu Lee, Arum Oh, Heon-Seok Han
Int J Thyroidol. 2023;16(1):128-133. doi: 10.11106/ijt.2023.16.1.128.
Reference
-
References
1. Grasberger H, Refetoff S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr. 2011; 23:421–8.
Article2. Fisher DA, Dussault JH, Foley TP Jr, Klein AH, LaFranchi S, Larsen PR, et al. Screening for congenital hypothyroidism: results of screening one million North American infants. J Pediatr. 1979; 94:700–5.
Article3. LaFranchi SH. Increasing incidence of congenital hypothyroidism: some answers, more questions. J Clin Endocrinol Metab. 2011; 96:2395–7.4. Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010; 5:17.
Article5. Szinnai G. Clinical genetics of congenital hypothyroidism. Endocr Dev. 2014; 26:60–78.
Article6. Nicholas AK, Serra EG, Cangul H, Alyaarubi S, Ullah I, Schoenmakers E, et al. Comprehensive screening of eight known causative genes in congenital hypothyroidism with gland-in-situ. J Clin Endocrinol Metab. 2016; 101:4521–31.
Article7. Muzza M, Rabbiosi S, Vigone MC, Zamproni I, Cirello V, Maffini MA, et al. The clinical and molecular characterization of patients with dyshormonogenic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J Clin Endocrinol Metab. 2014; 99:E544–53.
Article8. Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014; 99:363–84.
Article9. Spitzweg C, Heufelder AE, Morris JC. Thyroid iodine transport. Thyroid. 2000; 10:321–30.
Article10. Knobel M, Medeiros-Neto G. An outline of inherited disorders of the thyroid hormone generating system. Thyroid. 2003; 13:771–801.
Article11. Targovnik HM, Esperante SA, Rivolta CM. Genetics and phenomics of hypothyroidism and goiter due to thyroglobulin mutations. Mol Cell Endocrinol. 2010; 322:44–55.
Article12. Muzza M, Fugazzola L. Disorders of H(2)O(2) generation. Best Pract Res Clin Endocrinol Metab. 2017; 31:225–40.13. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000; 275:23227–33.
Article14. Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006; 281:18269–72.15. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008; 93:605–10.
Article16. Moreno JC. Identification of novel genes involved in congenital hypothyroidism using serial analysis of gene expression. Horm Res. 2003; 60 Suppl 3:96–102.
Article17. Portulano C, Paroder-Belenitsky M, Carrasco N. The Na+/I- symporter (NIS): mechanism and medical impact. Endocr Rev. 2014; 35:106–49.18. Stanbury JB, Chapman EM. Congenital hypothyroidism with goitre. Absence of an iodide-concentrating mechanism. Lancet. 1960; 1:1162–5.
Article19. Fujiwara H, Tatsumi K, Miki K, Harada T, Miyai K, Takai S, et al. Congenital hypothyroidism caused by a mutation in the Na+/I- symporter. Nat Genet. 1997; 16:124–5.
Article20. The Human Gene Mutation Database [Internet]. Cardiff (UK): Cardiff University;2015. [cited 2018 Jul 23]. Available from: http://www.hgmd.cf.ac.uk.21. Hannoush ZC, Weiss RE. Defects of thyroid hormone synthesis and action. Endocrinol Metab Clin North Am. 2017; 46:375–88.
Article22. Szinnai G, Kosugi S, Derrien C, Lucidarme N, David V, Czernichow P, et al. Extending the clinical heterogeneity of iodide transport defect (ITD): a novel mutation R124H of the sodium/iodide symporter gene and review of genotypephenotype correlations in ITD. J Clin Endocrinol Metab. 2006; 91:1199–204.
Article23. Everett LA, Green ED. A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet. 1999; 8:1883–91.24. Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997; 17:411–22.
Article25. Banghova K, Al Taji E, Cinek O, Novotna D, Pourova R, Zapletalova J, et al. Pendred syndrome among patients with congenital hypothyroidism detected by neonatal screening: identification of two novel PDS/SLC26A4 mutations. Eur J Pediatr. 2008; 167:777–83.
Article26. Reardon W, Trembath RC. Pendred syndrome. J Med Genet. 1996; 33:1037–40.
Article27. Ladsous M, Vlaeminck-Guillem V, Dumur V, Vincent C, Dubrulle F, Dhaenens CM, et al. Analysis of the thyroid phenotype in 42 patients with Pendred syndrome and nonsyndromic enlargement of the vestibular aqueduct. Thyroid. 2014; 24:639–48.
Article28. Targovnik HM, Citterio CE, Rivolta CM. Iodide handling disorders (NIS, TPO, TG, IYD). Best Pract Res Clin Endocrinol Metab. 2017; 31:195–212.
Article29. Ieiri T, Cochaux P, Targovnik HM, Suzuki M, Shimoda S, Perret J, et al. A 3' splice site mutation in the thyroglobulin gene responsible for congenital goiter with hypothyroidism. J Clin Invest. 1991; 88:1901–5.
Article30. Medeiros-Neto G, Kim PS, Yoo SE, Vono J, Targovnik HM, Camargo R, et al. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest. 1996; 98:2838–44.
Article31. Abramowicz MJ, Targovnik HM, Varela V, Cochaux P, Krawiec L, Pisarev MA, et al. Identification of a mutation in the coding sequence of the human thyroid peroxidase gene causing congenital goiter. J Clin Invest. 1992; 90:1200–4.
Article32. Ris-Stalpers C, Bikker H. Genetics and phenomics of hypothyroidism and goiter due to TPO mutations. Mol Cell Endocrinol. 2010; 322:38–43.
Article33. Fugazzola L, Cerutti N, Mannavola D, Vannucchi G, Fallini C, Persani L, et al. Monoallelic expression of mutant thyroid peroxidase allele causing total iodide organification defect. J Clin Endocrinol Metab. 2003; 88:3264–71.
Article34. Kotani T, Umeki K, Yamamoto I, Ohtaki S, Adachi M, Tachibana K. Iodide organification defects resulting from cosegregation of mutated and null thyroid peroxidase alleles. Mol Cell Endocrinol. 2001; 182:61–8.
Article35. Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2009; 23:1205–18.
Article36. Moreno JC, Bikker H, Kempers MJ, van Trotsenburg AS, Baas F, de Vijlder JJ, et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002; 347:95–102.
Article37. Maruo Y, Takahashi H, Soeda I, Nishikura N, Matsui K, Ota Y, et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J Clin Endocrinol Metab. 2008; 93:4261–7.
Article38. Enacán RE, Masnata ME, Belforte F, Papendieck P, Olcese MC, Siffo S, et al. Transient congenital hypothyroidism due to biallelic defects of DUOX2 gene. Two clinical cases. Arch Argent Pediatr. 2017; 115:e162–5.39. Wang F, Lu K, Yang Z, Zhang S, Lu W, Zhang L, et al. Genotypes and phenotypes of congenital goitre and hypothyroidism caused by mutations in dual oxidase 2 genes. Clin Endocrinol (Oxf). 2014; 81:452–7.
Article40. Jin HY, Heo SH, Kim YM, Kim GH, Choi JH, Lee BH, et al. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediatr. 2014; 82:252–60.
Article41. Matsuo K, Tanahashi Y, Mukai T, Suzuki S, Tajima T, Azuma H, et al. High prevalence of DUOX2 mutations in Japanese patients with permanent congenital hypothyroidism or transient hypothyroidism. J Pediatr Endocrinol Metab. 2016; 29:807–12.
Article42. Vigone MC, Fugazzola L, Zamproni I, Passoni A, Di Candia S, Chiumello G, et al. Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum Mutat. 2005; 26:395.
Article43. Liu S, Liu L, Niu X, Lu D, Xia H, Yan S. A novel missense mutation (I26M) in DUOXA2 causing congenital goiter hypothyroidism impairs NADPH oxidase activity but not protein expression. J Clin Endocrinol Metab. 2015; 100:1225–9.44. Gnidehou S, Caillou B, Talbot M, Ohayon R, Kaniewski J, Noël-Hudson MS, et al. Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site. FASEB J. 2004; 18:1574–6.
Article45. Moreno JC, Klootwijk W, van Toor H, Pinto G, D'Alessandro M, Lèger A, et al. Mutations in the iodotyrosine deiodinase gene and hypothyroidism. N Engl J Med. 2008; 358:1811–8.
Article46. Afink G, Kulik W, Overmars H, de Randamie J, Veenboer T, van Cruchten A, et al. Molecular characterization of iodotyrosine dehalogenase deficiency in patients with hypothyroidism. J Clin Endocrinol Metab. 2008; 93:4894–901.
Article47. Keller-Petrot I, Leger J, Sergent-Alaoui A, de Labriolle-Vaylet C. Congenital hypothyroidism: role of nuclear medicine. Semin Nucl Med. 2017; 47:135–42.
Article48. Leslie WD. Thyroid scintigraphy and perchlorate discharge test in the diagnosis of congenital hypothyroidism. Eur J Nucl Med. 1996; 23:230.
Article49. Cavarzere P, Castanet M, Polak M, Raux-Demay MC, Cabrol S, Carel JC, et al. Clinical description of infants with congenital hypothyroidism and iodide organification defects. Horm Res. 2008; 70:240–8.
Article50. Sun F, Zhang JX, Yang CY, Gao GQ, Zhu WB, Han B, et al. The genetic characteristics of congenital hypothyroidism in China by comprehensive screening of 21 candidate genes. Eur J Endocrinol. 2018; 178:623–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Resistance Syndrome to Thyroid Hormone Associated with Mutation (G345D) in the Thyroid Hormone Receptor Beta Gene
- CCAAT-binding Transcription Factor(CTF) Proteins Required for the Transcriptional Stimulation by Thyroid Hormone in GH4C1 Cells
- A Case of Resistance to Thyroid Hormone with Thyroid Cancer
- Resistance to thyroid hormone due to a novel mutation of thyroid hormone receptor beta gene
- A Newly Identified Insertion Mutation in the Thyroid Hormone Receptor-beta Gene in a Korean Family with Generalized Thyroid Hormone Resistance