Clin Exp Otorhinolaryngol.
2023 May;16(2):148-158. 10.21053/ceo.2022.01718.
Efficacy of Steroid-Impregnated Spacers After Endoscopic Sinus Surgery in Chronic Rhinosinusitis: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Otolaryngology-Head and Neck Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Surgery, College of Medicine, Taif University, Taif, Saudi Arabia
- KMID: 2542356
- DOI: http://doi.org/10.21053/ceo.2022.01718
Abstract
Objectives
. The aim of this study was to compare the effect of steroid-impregnated spacers to that of conventional management after endoscopic sinus surgery (ESS) in patients with chronic rhinosinusitis (CRS).
Methods
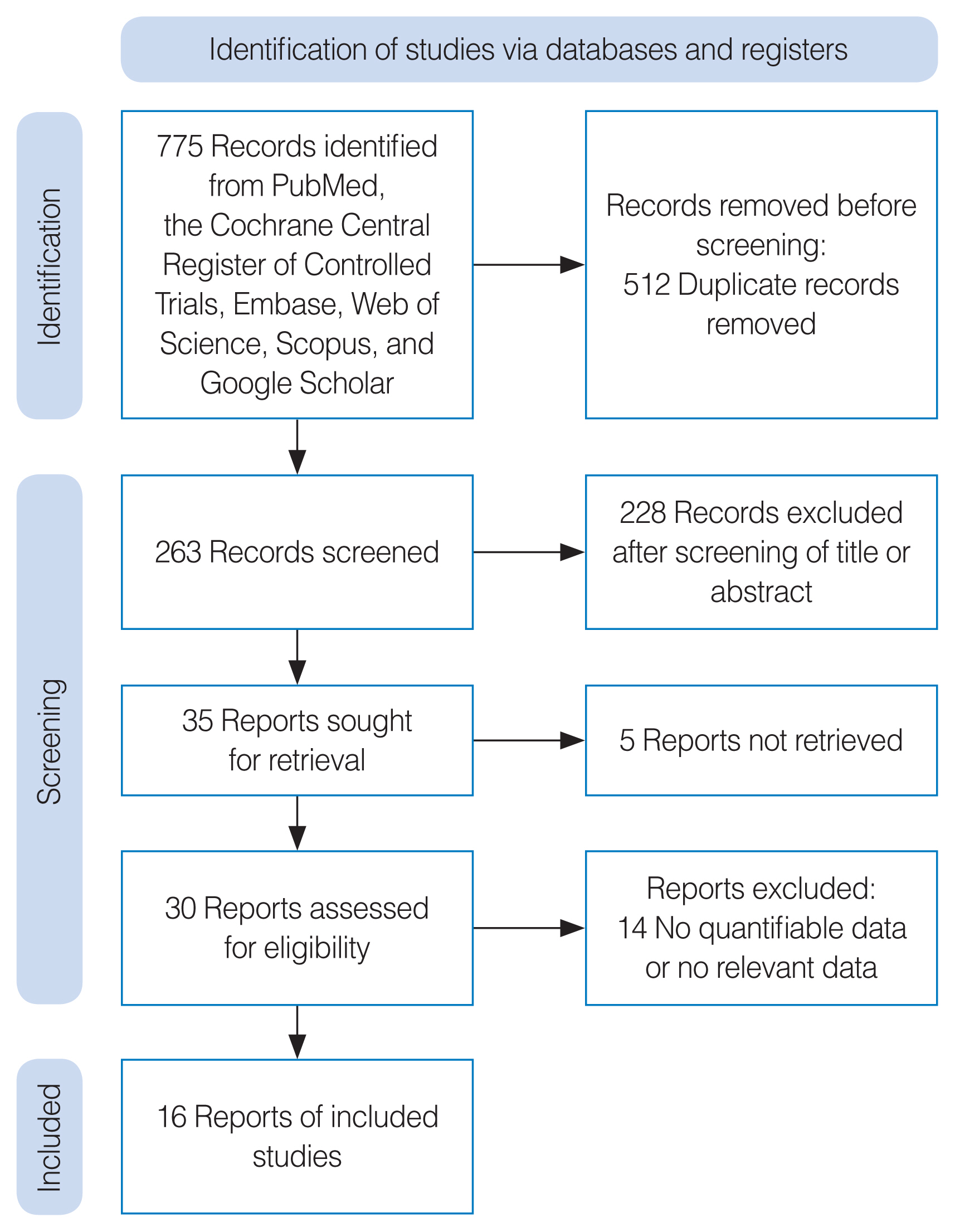
. Six databases were searched from inception until November 2022. Sixteen studies were found that compared the improvement of chronic sinusitis-related symptoms and postoperative outcomes between a steroid-impregnated spacer group and a control group (non-steroid-impregnated spacers). The Cochrane risk of bias tool (for randomized controlled studies) and the Newcastle-Ottawa Scale (for non-randomized controlled studies) were used to assess the quality of the works included.
Results
. Regarding the endoscopic findings, the degree of mucosal edema, ethmoid inflammation, crust formation at 2–3 months postoperatively, nasal discharge, polyposis, and scarring/synechia were significantly lower in the steroid-impregnated spacer group. The steroid-impregnated spacer group also showed significantly lower Lund–Kennedy scores and perioperative sinus endoscopy scores than the control group at 2–3 weeks postoperatively. Furthermore, the steroid-impregnated spacer group had lower rates of adhesions, middle turbinate lateralization, polypoid changes, the need for oral steroid use, the need for postoperative therapeutic interventions, and lysis of adhesions than controls. However, no significant between-group differences were found in short-term (2–3 weeks postoperatively) endoscopic findings regarding nasal discharge, postoperative crusting, polyposis, or scarring/synechia.
Conclusion
. Steroid-impregnated nasal packing reduced the rates of postoperative intervention and recurrent polyposis and inflammation in CRS patients undergoing ESS.
Keyword
Figure
Cited by 2 articles
-
Efficacy of Platelet-Rich Plasma in the Treatment of Persistent Olfactory Impairment After COVID-19: A Systematic Review and Meta-Analysis
Ah Young Bae, Do Hyun Kim, Se Hwan Hwang
J Rhinol. 2024;31(1):1-7. doi: 10.18787/jr.2024.00006.Perinatal Risk Factors for Asthma and Allergic Rhinitis in Children and Adolescents
Se Hwan Hwang, Hyesoo Shin, Gulnaz Stybayeva, Do Hyun Kim
Clin Exp Otorhinolaryngol. 2024;17(2):168-176. doi: 10.21053/ceo.2024.00024.
Reference
-
1. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020; Feb. 58(Suppl 29):1–464.2. Park DY, Choi JH, Kim DK, Jung YG, Mun SJ, Min HJ, et al. Clinical practice guideline: nasal irrigation for chronic rhinosinusitis in adults. Clin Exp Otorhinolaryngol. 2022; Feb. 15(1):5–23.
Article3. Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021; Mar. 11(3):213–739.4. Svider PF, Sekhsaria V, Cohen DS, Eloy JA, Setzen M, Folbe AJ. Geographic and temporal trends in frontal sinus surgery. Int Forum Allergy Rhinol. 2015; Jan. 5(1):46–54.
Article5. DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017; Mar. 127(3):550–5.
Article6. Stein NR, Jafari A, DeConde AS. Revision rates and time to revision following endoscopic sinus surgery: a large database analysis. Laryngoscope. 2018; Jan. 128(1):31–6.
Article7. Cote DW, Wright ED. Triamcinolone-impregnated nasal dressing following endoscopic sinus surgery: a randomized, double-blind, placebo-controlled study. Laryngoscope. 2010; Jun. 120(6):1269–73.
Article8. Adriaensen GF, Lim KH, Fokkens WJ. Safety and efficacy of a bioabsorbable fluticasone propionate-eluting sinus dressing in postoperative management of endoscopic sinus surgery: a randomized clinical trial. Int Forum Allergy Rhinol. 2017; Aug. 7(8):813–20.
Article9. Grzeskowiak B, Wierzchowska M, Walorek R, Seredyka-Burduk M, Wawrzyniak K, Burduk PK. Steroid vs. antibiotic impregnated absorbable nasal packing for wound healing after endoscopic sinus surgery: a randomized, double blind, placebo-controlled study. Braz J Otorhinolaryngol. 2019; Jul–Aug. 85(4):473–80.
Article10. Gyawali BR, Pradhan B, Thapa N. Comparison of outcomes of triamcinolone versus normal saline soaked polyvinyl alcohol pack following bilateral endoscopic sinus surgery. Rhinology. 2019; Aug. 57(4):287–92.
Article11. Ha T, Valentine R, Moratti S, Hanton L, Robinson S, Wormald PJ. The efficacy of a novel budesonide chitosan gel on wound healing following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2018; Mar. 8(3):435–43.
Article12. Huang Z, Zhou B, Wang D, Zang H, Zhang H, Wang H, et al. Comparison of bioabsorbable steroid-eluting sinus stents versus nasopore after endoscopic sinus surgery: a multicenter, randomized, controlled, single-blinded clinical trial. Ear Nose Throat J. 2022; May. 101(4):260–7.
Article13. Hwang CS, Al Sharhan SS, Kim BR, Kim SI, Kim JW, Cho HJ, et al. Randomized controlled trial of steroid-soaked absorbable calcium alginate nasal packing following endoscopic sinus surgery. Laryngoscope. 2018; Feb. 128(2):311–6.
Article14. Luong A, Ow RA, Singh A, Weiss RL, Han JK, Gerencer R, et al. Safety and effectiveness of a bioabsorbable steroid-releasing implant for the paranasal sinus ostia: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2018; Jan. 144(1):28–35.15. Marple BF, Smith TL, Han JK, Gould AR, Jampel HD, Stambaugh JW, et al. Advance II: a prospective, randomized study assessing safety and efficacy of bioabsorbable steroid-releasing sinus implants. Otolaryngol Head Neck Surg. 2012; Jun. 146(6):1004–11.16. More Y, Willen S, Catalano P. Management of early nasal polyposis using a steroid-impregnated nasal dressing. Int Forum Allergy Rhinol. 2011; Sep–Oct. 1(5):401–4.
Article17. Murr AH, Smith TL, Hwang PH, Bhattacharyya N, Lanier BJ, Stambaugh JW, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent. Int Forum Allergy Rhinol. 2011; Jan–Feb. 1(1):23–32.
Article18. Rudmik L, Mace J, Mechor B. Effect of a dexamethasone Sinu-Foam-TM middle meatal spacer on endoscopic sinus surgery outcomes: a randomized, double-blind, placebo-controlled trial. Int Forum Allergy Rhinol. 2012; May–Jun. 2(3):248–51.
Article19. Sabarinath V, Harish MR, Divakaran S. Triamcinolone impregnated nasal pack in endoscopic sinus surgery: our experience. Indian J Otolaryngol Head Neck Surg. 2017; Mar. 69(1):88–92.
Article20. Samarei R, Rasouli J, Mehdikhani F. Efficacy of triamcinolone acetonide-impregnated Gelfoam nasal pack in management of chronic sinusitis with nasal polyps following endoscopic sinus surgery: a perfectly matched, placebo-controlled trial study. Eur Arch Otorhinolaryngol. 2022; Jun. 279(6):2915–24.
Article21. Smith TL, Singh A, Luong A, Ow RA, Shotts SD, Sautter NB, et al. Randomized controlled trial of a bioabsorbable steroid-releasing implant in the frontal sinus opening. Laryngoscope. 2016; Dec. 126(12):2659–64.
Article22. Xu J, Park SJ, Park HS, Han R, Rha KS, Kim YM. Effects of triamcinolone-impregnated nasal dressing on subjective and objective outcomes following endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2016; Dec. 273(12):4351–7.
Article23. Zhao KQ, Yu YQ, Yu HM. Effects of mometasone furoate-impregnated biodegradable nasal dressing on endoscopic appearance in healing process following endoscopic sinus surgery: a randomized, double-blind, placebo-controlled study. Int Forum Allergy Rhinol. 2018; Nov. 8(11):1233–41.
Article24. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022; Aug. 15(3):230–46.
Article25. Hwang SH, Kim JS, Choi BY, Kim JK, Kim BG. Practical review of olfactory training and COVID-19. J Rhinol. 2022; 29(3):127–33.
Article26. Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope. 2007; Nov. 117(11 Pt 2 Suppl 115):1–28.
Article27. Li W, Lu H, Wang H, Sun X, Wang D. Efficacy and safety of steroid-impregnated implants following sinus surgery: a meta-analysis. Laryngoscope. 2020; Dec. 130(12):2754–9.
Article28. Stefan MM, Rabie NA, Sobhy TS, Maarouf AM. Effect of steroid-releasing sinus implants after endoscopic sinus surgery (ESS) on postoperative outcomes: a meta-analytical study. Egypt J Otolaryngol. 2019; Jul. 35:250–5.
Article29. Zhao X, Grewal A, Briel M, Lee JM. A systematic review of nonabsorbable, absorbable, and steroid-impregnated spacers following endoscopic sinus surgery. Int Forum Allergy Rhinol. 2013; Nov. 3(11):896–904.
Article30. Goshtasbi K, Abouzari M, Abiri A, Yasaka T, Sahyouni R, Bitner B, et al. Efficacy of steroid-eluting stents in management of chronic rhinosinusitis after endoscopic sinus surgery: updated meta-analysis. Int Forum Allergy Rhinol. 2019; Dec. 9(12):1443–50.
Article31. Zhang M, Ryan PJ, Shashinder S. Efficacy of absorbable steroid-impregnated nasal packing in functional endoscopic sinus surgery for chronic rhinosinusitis: a systematic review. Laryngoscope. 2021; Aug. 131(8):1704–14.32. Hosemann W, Wigand ME, Gode U, Langer F, Dunker I. Normal wound healing of the paranasal sinuses: clinical and experimental investigations. Eur Arch Otorhinolaryngol. 1991; 248(7):390–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Triamcinolone-Impregnated Nasal Packing Following Endoscopic Sinus Surgery
- Balloon Sinuplasty
- The Predictive Factors of Olfactory Changes after Endoscopic Sinus Surgery
- Systematic Review and Meta-analysis in Digestive Cancer Research
- Medical treatment according to phenotypes of chronic rhinosinusitis