J Korean Neurosurg Soc.
2023 May;66(3):308-315. 10.3340/jkns.2023.0046.
Korean Brain Tumor Society Consensus Review for the Practical Recommendations on Glioma Management in Korea
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2542016
- DOI: http://doi.org/10.3340/jkns.2023.0046
Abstract
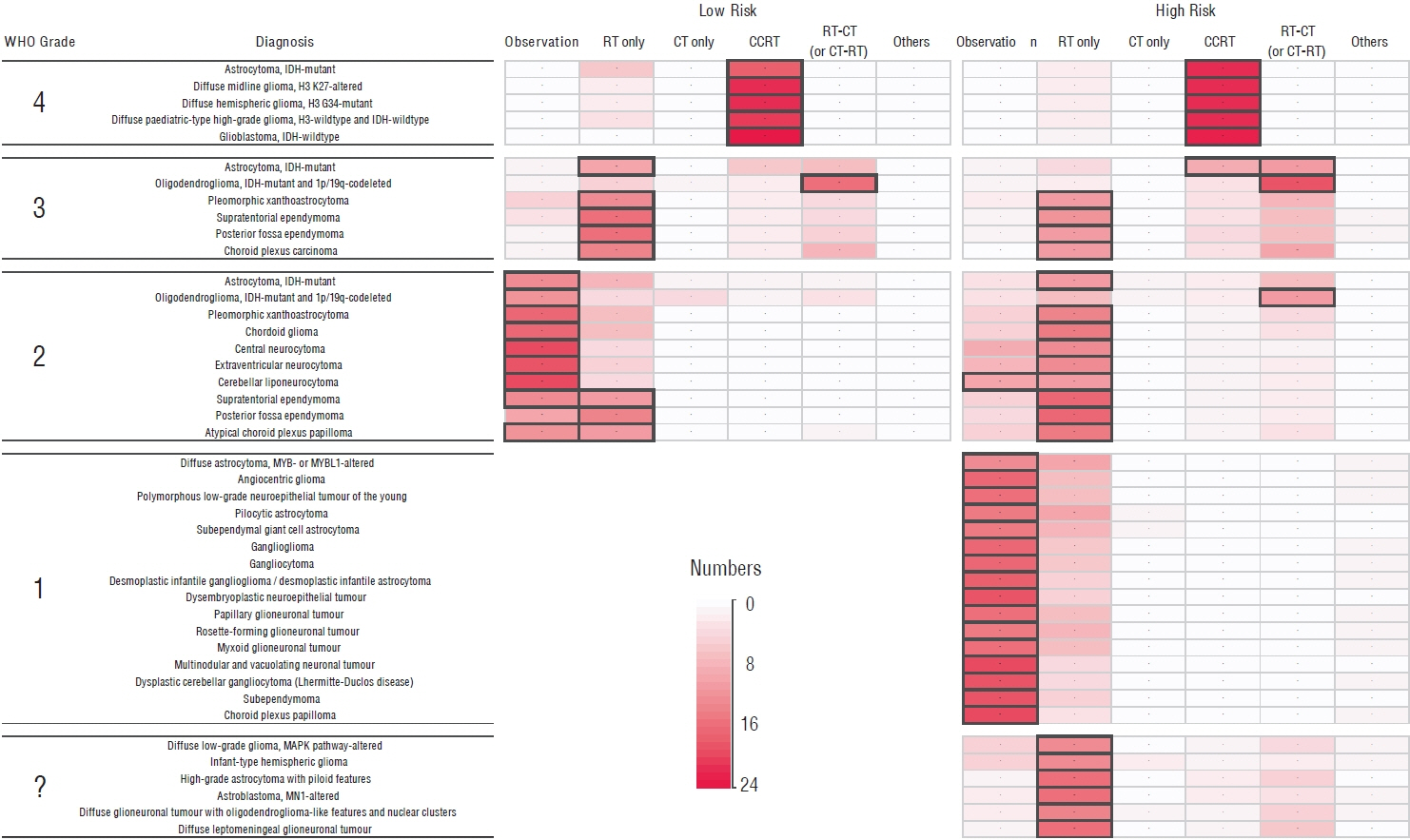
- Recent updates in genomic-integrated glioma classification have caused confusion in current clinical practice, as management protocols and health insurance systems are based on evidence from previous diagnostic classifications. The Korean Brain Tumor Society conducted an electronic questionnaire for society members, asking for their ideas on risk group categorization and preferred treatment for each individual diagnosis listed in the new World Health Organization (WHO) classification of gliomas. Additionally, the current off-label drug use (OLDU) protocols for glioma management approved by the Health Insurance Review and Assessment Service (HIRA) in Korea were investigated. A total of 24 responses were collected from 20 major institutes in Korea. A consensus was reached on the dichotomic definition of risk groups for glioma prognosis, using age, performance status, and extent of resection. In selecting management protocols, there was general consistency in decisions according to the WHO grade and the risk group, regardless of the individual diagnosis. As of December 2022, there were 22 OLDU protocols available for the management of gliomas in Korea. The consensus and available options described in this report will be temporarily helpful until there is an accumulation of evidence for effective management under the new classification system for gliomas.
Figure
Reference
-
References
1. Aguilera D, Castellino RC, Janss A, Schniederjan M, McNall R, MacDonald T, et al. Clinical responses of patients with diffuse leptomeningeal glioneuronal tumors to chemotherapy. Childs Nerv Syst. 34:329–334. 2018.
Article2. Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 374:1344–1355. 2016.
Article3. Bunevicius A, Sheehan JP. Radiosurgery for glioblastoma. Neurosurg Clin N Am. 32:117–128. 2021.
Article4. Dono A, Ballester LY, Primdahl D, Esquenazi Y, Bhatia A. IDH-mutant low-grade glioma: advances in molecular diagnosis, management, and future directions. Curr Oncol Rep. 23:20. 2021.
Article5. Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis†. Neurooncol Pract. 3:272–280. 2016.
Article6. Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, openlabel, phase 3 trial. Lancet. 393:678–688. 2019.
Article7. Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol. 85:e770–e781. 2012.
Article8. Jaeckle KA, Ballman KV, van den Bent M, Giannini C, Galanis E, Brown PD, et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 23:457–467. 2021.
Article9. Jakacki RI, Cohen KJ, Buxton A, Krailo MD, Burger PC, Rosenblum MK, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. 18:1442–1450. 2016.
Article10. Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAFV600-mutant gliomas: results from the VEBASKET study. J Clin Oncol. 36:3477–3484. 2018.
Article11. Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: a report of the RANO resect group. Neuro Oncol. 2022; [Epub ahead of print].12. Kim YZ, Kim CY, Lim DH. The overview of practical guidelines for gliomas by KSNO, NCCN, and EANO. Brain Tumor Res Treat. 10:83–93. 2022.
Article13. Kim YZ, Kim CY, Lim J, Sung KS, Lee J, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for glioblastomas: version 2018.01. Brain Tumor Res Treat. 7:1–9. 2019.
Article14. Kim YZ, Kim CY, Lim J, Sung KS, Lee J, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for WHO grade III cerebral gliomas in adults: version 2019.01. Brain Tumor Res Treat. 7:63–73. 2019.
Article15. Kim YZ, Kim CY, Wee CW, Roh TH, Hong JB, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for WHO grade II cerebral gliomas in adults: version 2019.01. Brain Tumor Res Treat. 7:74–84. 2019.
Article16. Lassman AB, Hoang-Xuan K, Polley MC, Brandes AA, Cairncross JG, Kros JM, et al. Joint final report of EORTC 26951 and RTOG 9402: phase III trials with procarbazine, lomustine, and vincristine chemotherapy for anaplastic oligodendroglial tumors. J Clin Oncol. 40:2539–2545. 2022.
Article17. Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 79:1487–1495. 2011.
Article18. Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 20:110–119. 2019.
Article19. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23:1231–1251. 2021.
Article20. Miller JJ, Gonzalez Castro LN, McBrayer S, Weller M, Cloughesy T, Portnow J, et al. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 25:4–25. 2023.
Article21. Mohile NA, Messersmith H, Gatson NT, Hottinger AF, Lassman A, Morton J, et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO-SNO guideline. J Clin Oncol. 40:403–426. 2022.
Article22. National Comprehensive Cancer Network : Central Nervous System Cancers (version 2.2022). Available at : https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425.23. Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 149:499–510. 2020.
Article24. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
Article25. Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 15:943–953. 2014.
Article26. van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 390:1645–1653. 2017.
Article27. van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 31:344–350. 2013.
Article28. van den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 22:813–823. 2021.
Article29. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021.
Article30. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 23:53–64. 2022.
Article31. Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 22:1073–1113. 2020.
Article32. WHO Classification of Tumours Editorial Board : Central Nervous System Tumours. Lyon : International Agency for Research on Cancer, 2021.33. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 377:1954–1963. 2017.
Article34. Yoon HI, Wee CW, Kim YZ, Seo Y, Im JH, Dho YS, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for adult diffuse midline glioma: version 2021.1. Brain Tumor Res Treat. 9:1–8. 2021.
Article35. Zhuang H, Yuan X, Zheng Y, Li X, Chang JY, Wang J, et al. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci Rep. 6:24364. 2016.
Article