Intest Res.
2023 Apr;21(2):252-265. 10.5217/ir.2022.00092.
Risks of colorectal cancer and biliary cancer according to accompanied primary sclerosing cholangitis in Korean patients with ulcerative colitis: a nationwide population-based study
- Affiliations
-
- 1Department of Gastroenterology, Hanyang University Guri Hospital, Guri, Korea
- 2Department of Clinical Epidemiology and Biostatistics, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Gastroenterology, Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea
- 4Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2541898
- DOI: http://doi.org/10.5217/ir.2022.00092
Abstract
- Background/Aims
We conducted a nationwide population-based study to investigate incidence rates of colorectal and biliary cancers according to accompanying primary sclerosing cholangitis in Korean ulcerative colitis patients.
Methods
We used the Health Insurance Review and Assessment claim database from January 2007 to April 2020. Standardized incidence ratios of colorectal and biliary cancers in ulcerative colitis patients were calculated.
Results
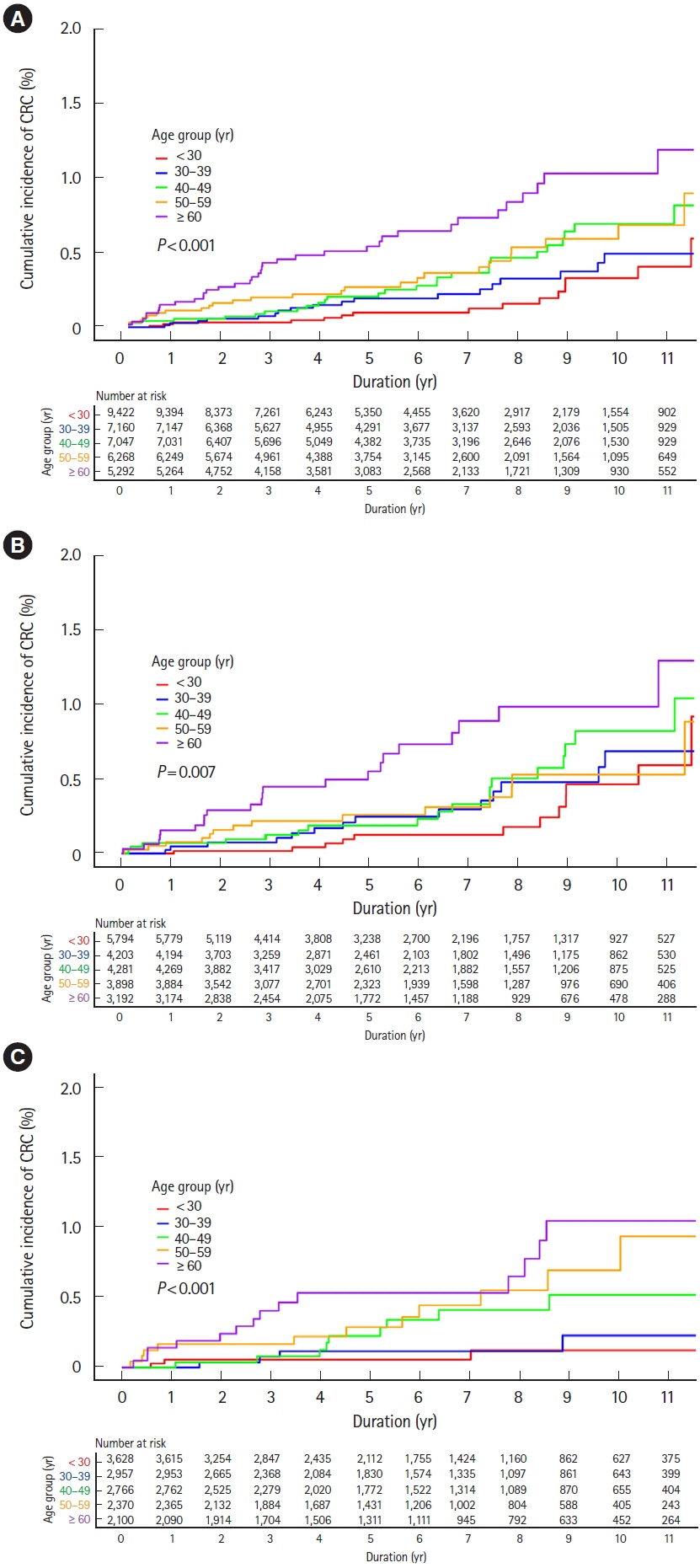
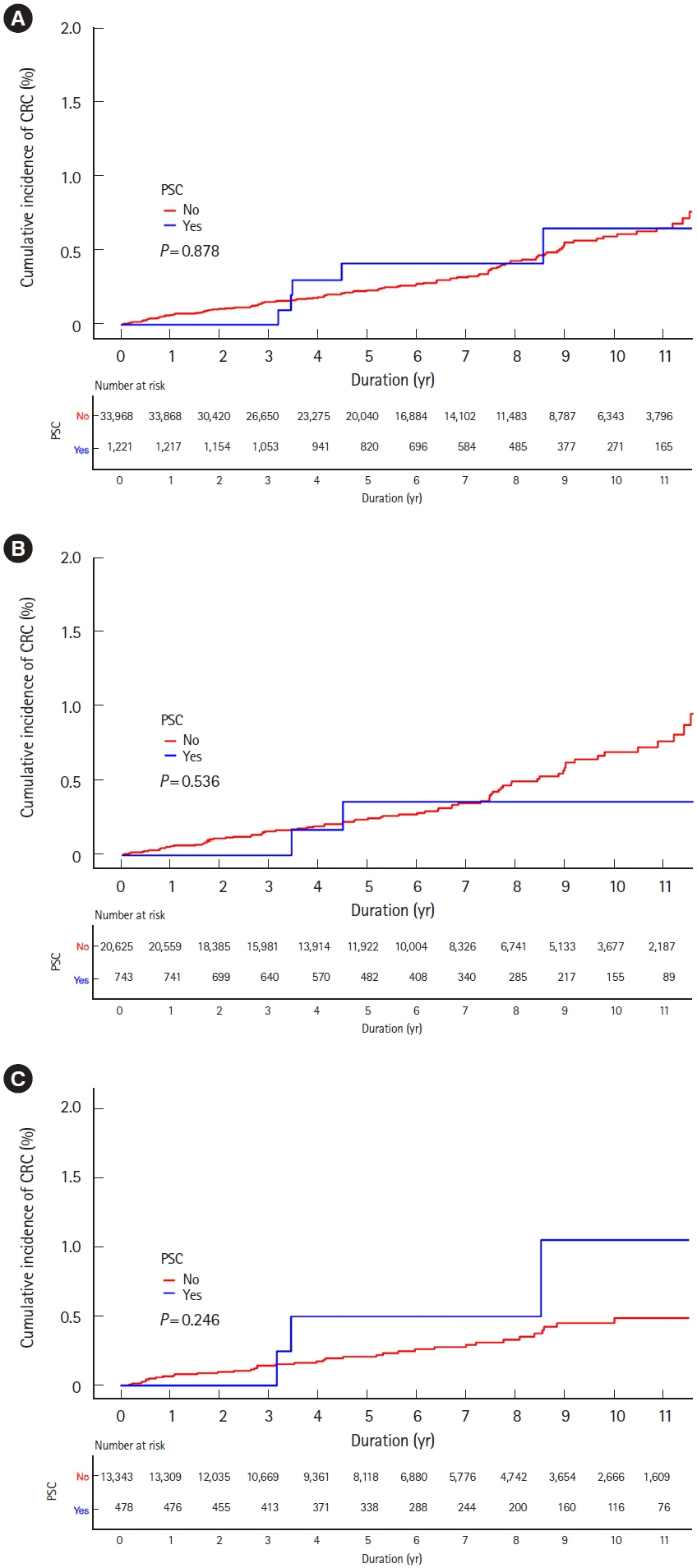
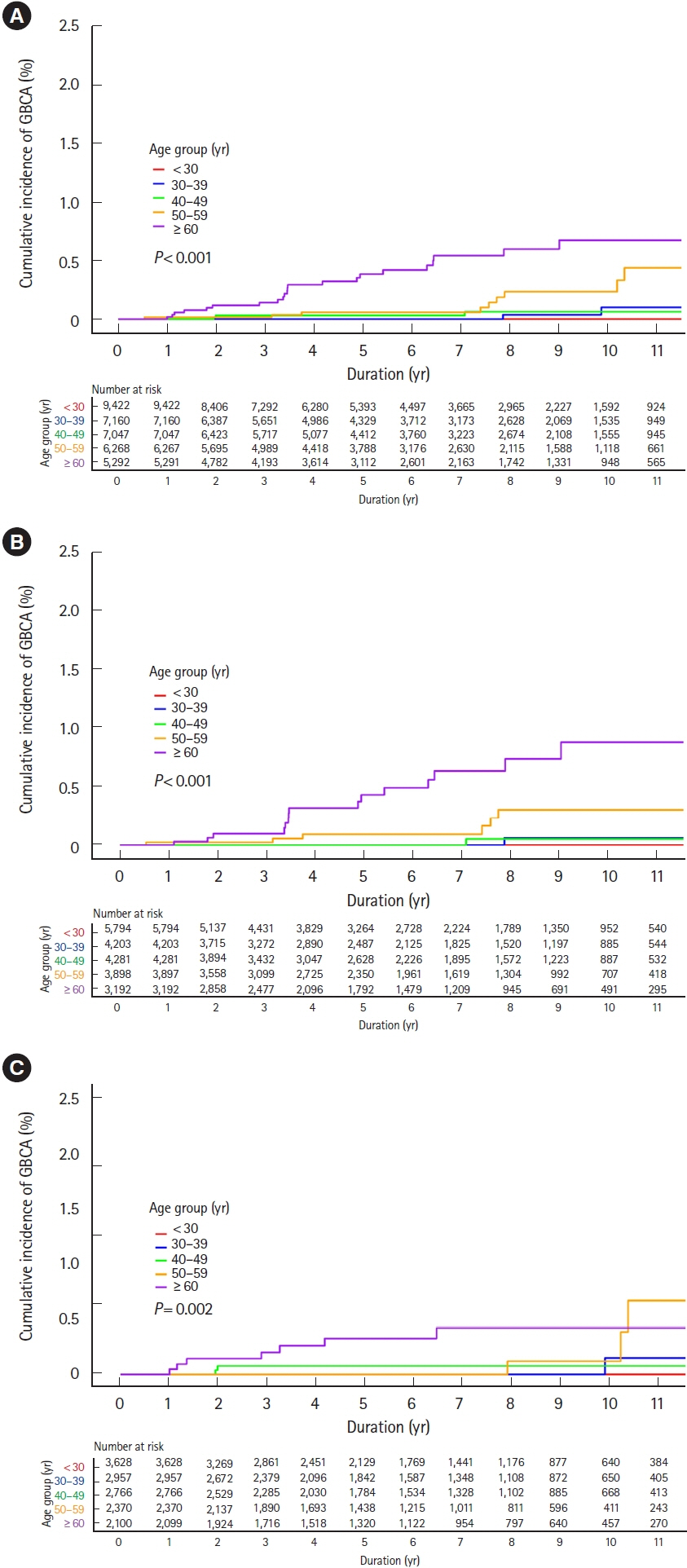
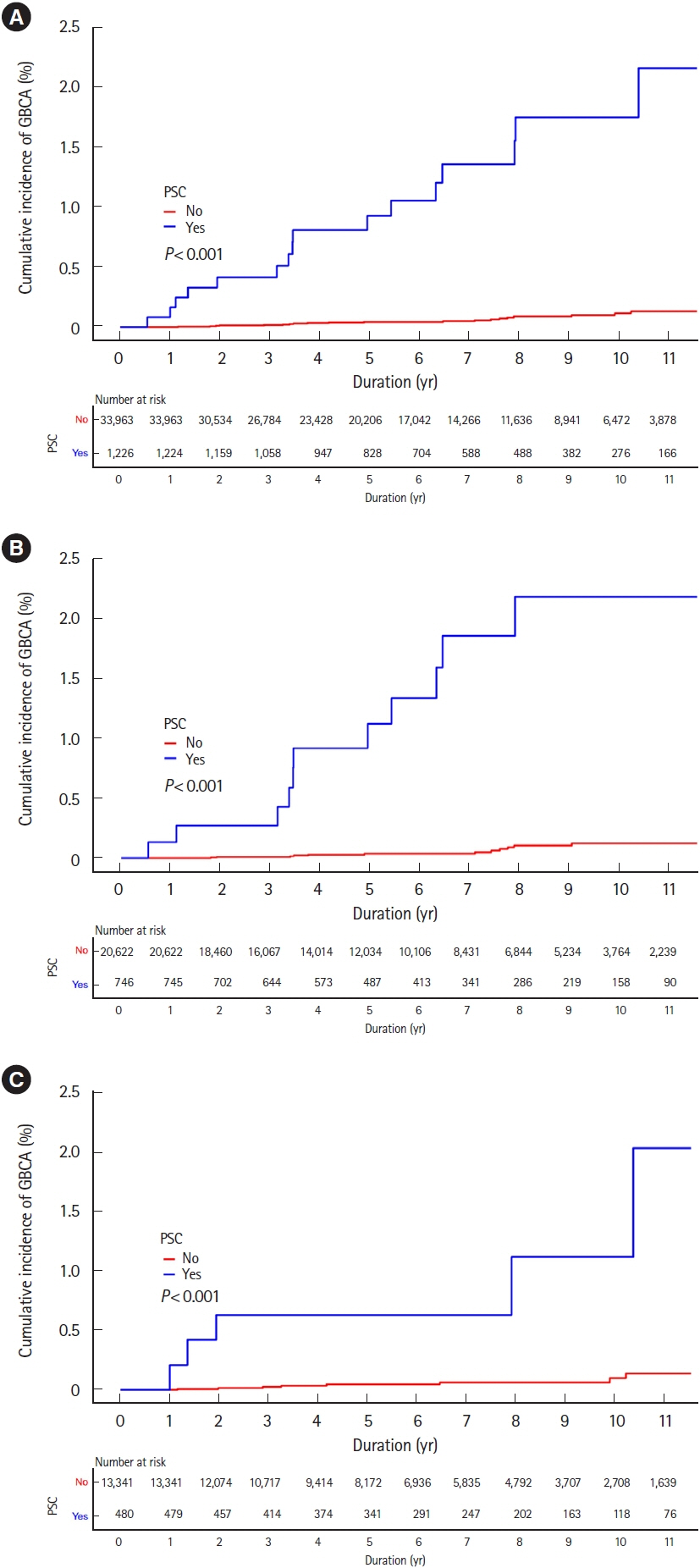
Among 35,189 newly diagnosed ulcerative colitis patients, 1,224 patients were diagnosed with primary sclerosing cholangitis. During the study period, 122 and 52 patients were diagnosed with colorectal and biliary cancers, respectively. Incidences of colorectal cancer were not higher in ulcerative colitis patients than those in the general population (standardized incidence ratios, 0.83; 95% confidence interval, 0.69–0.99), regardless of accompanied primary sclerosing cholangitis (standardized incidence ratio, 0.73; 95% confidence interval, 0.24–1.71). While incidences of biliary cancer were not higher in ulcerative colitis patients than those in the general population (standardized incidence ratio, 1.14; 95% confidence interval, 0.80–1.58), these were much higher with accompanied primary sclerosing cholangitis (standardized incidence ratio, 10.07; 95% confidence interval, 5.75–16.36). Cumulative incidences of colorectal and biliary cancers increased in patients who were diagnosed with ulcerative colitis at an older age.
Conclusions
In Korean ulcerative colitis patients, colorectal cancer incidences were not higher than those in the general population regardless of accompanied primary sclerosing cholangitis. However, biliary cancer incidences were much higher in ulcerative colitis patients with primary sclerosing cholangitis than in those without, or in the general population.
Figure
Cited by 2 articles
-
Are the risks of colorectal cancer and biliary cancer really increased if patients with ulcerative colitis have primary sclerosing cholangitis?
Jung Wook Lee, Won Moon
Intest Res. 2023;21(2):171-173. doi: 10.5217/ir.2023.00049.Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
You Sun Kim, Edward H. Hurley, Yoojeong Park, Sungjin Ko
Intest Res. 2023;21(4):420-432. doi: 10.5217/ir.2023.00039.
Reference
-
1. Kim JM, Cheon JH. Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases. Intest Res. 2020; 18:249–264.
Article2. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013; 382:1587–1599.
Article3. Tanaka A, Mertens JC. Ulcerative colitis with and without primary sclerosing cholangitis: two different diseases? Inflamm Intest Dis. 2016; 1:9–14.
Article4. Ye BD, Yang SK, Boo SJ, et al. Clinical characteristics of ulcerative colitis associated with primary sclerosing cholangitis in Korea. Inflamm Bowel Dis. 2011; 17:1901–1906.
Article5. de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015; 21:1956–1971.
Article6. Yashiro M. Ulcerative colitis-associated colorectal cancer. World J Gastroenterol. 2014; 20:16389–16397.
Article7. Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011; 34:497–508.
Article8. Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009; 15:3329–3340.
Article9. Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011; 54:1842–1852.
Article10. Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004; 99:523–526.
Article11. Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis: a population-based study. Scand J Gastroenterol. 1997; 32:1042–1045.
Article12. Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017; 32:718–728.
Article13. Kim HJ, Hann HJ, Hong SN, et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm Bowel Dis. 2015; 21:623–630.
Article14. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–430.
Article15. Song EM, Lee HS, Kim YJ, et al. Incidence and clinical impact of perianal disease in patients with ulcerative colitis: a nationwide population-based study. J Gastroenterol Hepatol. 2019; 34:1011–1017.
Article16. Song EM, Lee HS, Kim YJ, et al. Incidence and outcomes of perianal disease in an Asian population with Crohn’s disease: a nationwide population-based study. Dig Dis Sci. 2020; 65:1189–1196.
Article17. Cho MS, Yun JE, Park JJ, et al. Outcomes after use of standardand low-dose non-vitamin K oral anticoagulants in Asian patients with atrial fibrillation. Stroke. 2019; 50:110–118.
Article18. Targownik LE, Singh H, Nugent Z, Bernstein CN. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012; 107:1228–1235.
Article19. Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2014; 21:43–50.
Article20. Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol. 2006; 6:244–251.
Article21. Khosravi Khorashad A, Khajedaluee M, Mokhtari Amirmajdi E, et al. Frequency and risk factors of primary sclerosing cholangitis among patients with inflammatory bowel disease in North-East of Iran. Gastroenterol Hepatol Bed Bench. 2015; 8:200–206.22. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001; 48:526–535.
Article23. Söderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009; 136:1561–1567.
Article24. Olén O, Erichsen R, Sachs MC, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020; 395:123–131.
Article25. Chambers WM, Warren BF, Jewell DP, Mortensen NJ. Cancer surveillance in ulcerative colitis. Br J Surg. 2005; 92:928–936.
Article26. Nam S, Choi YJ, Kim DW, Park EC, Kang JG. Risk factors for colorectal cancer in Korea: a population-based retrospective cohort study. Ann Coloproctol. 2019; 35:347–356.
Article27. Høydahl Ø, Edna TH, Xanthoulis A, Lydersen S, Endreseth BH. Long-term trends in colorectal cancer: incidence, localization, and presentation. BMC Cancer. 2020; 20:1077.
Article28. Benson VS, Patnick J, Davies AK, et al. Colorectal cancer screening: a comparison of 35 initiatives in 17 countries. Int J Cancer. 2008; 122:1357–1367.
Article29. Issa IA, Noureddine M. Colorectal cancer screening: an updated review of the available options. World J Gastroenterol. 2017; 23:5086–5096.
Article30. Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010; 375:1624–1633.
Article31. Choi PM, Nugent FW, Schoetz DJ Jr, Silverman ML, Haggitt RC. Colonoscopic surveillance reduces mortality from colorectal cancer in ulcerative colitis. Gastroenterology. 1993; 105:418–424.
Article32. Velayos FS, Loftus EV Jr, Jess T, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: a case-control study. Gastroenterology. 2006; 130:1941–1949.
Article33. Oh SJ, Shin GY, Soh H, et al. Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis. Intest Res. 2021; 19:323–331.
Article34. Hisamatsu T, Kim HJ, Motoya S, et al. Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI). Intest Res. 2021; 19:386–397.
Article35. Broomé U, Löfberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995; 22:1404–1408.
Article36. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002; 56:48–54.
Article37. Magro F, Gionchetti P, Eliakim R, et al. Third European evidencebased consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article38. Yusoff AR, Razak MM, Yoong BK, Vijeyasingam R, Siti ZM. Survival analysis of cholangiocarcinoma: a 10-year experience in Malaysia. World J Gastroenterol. 2012; 18:458–465.
Article39. Scharl S, Barthel C, Rossel JB, et al. Malignancies in Inflammatory bowel disease: frequency, incidence and risk factors-results from the Swiss IBD Cohort Study. Am J Gastroenterol. 2019; 114:116–126.
Article40. Song SO, Lee YH, Kim DW, et al. Trends in diabetes incidence in the last decade based on Korean National Health Insurance claims data. Endocrinol Metab (Seoul). 2016; 31:292–299.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Are the risks of colorectal cancer and biliary cancer really increased if patients with ulcerative colitis have primary sclerosing cholangitis?
- Primary Sclerosing Cholangitis in Patients with Ulcerative Colitis: Two Case Reports
- A Case of Cholangiocarcinoma and Colorectal Cancer Diagnosed Simultaneously in a Patient with Ulcerative Colitis and Concurrent Primary Sclerosing Cholangitis
- Recent Advances in Understanding Colorectal Cancer and Dysplasia Related to Ulcerative Colitis
- Two Cases of Atypical Ulcerative Colitis - Rectal Sparing Ulcerative Colitis Associated with Primary Sclerosing Cholangitis -