Ann Rehabil Med.
2023 Apr;47(2):138-146. 10.5535/arm.22152.
Clinical and Swallowing Characteristics Related With Respiratory Infection in Parkinsonism Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Rehabilitation Medicine, Seongbuk Seoul Convalescent Hospital, Seoul, Korea
- 3National Traffic Injury Rehabilitation Hospital, Yangpyeong, Korea
- 4Institute on Aging, Seoul National University, Seoul, Korea
- KMID: 2541847
- DOI: http://doi.org/10.5535/arm.22152
Abstract
Objective
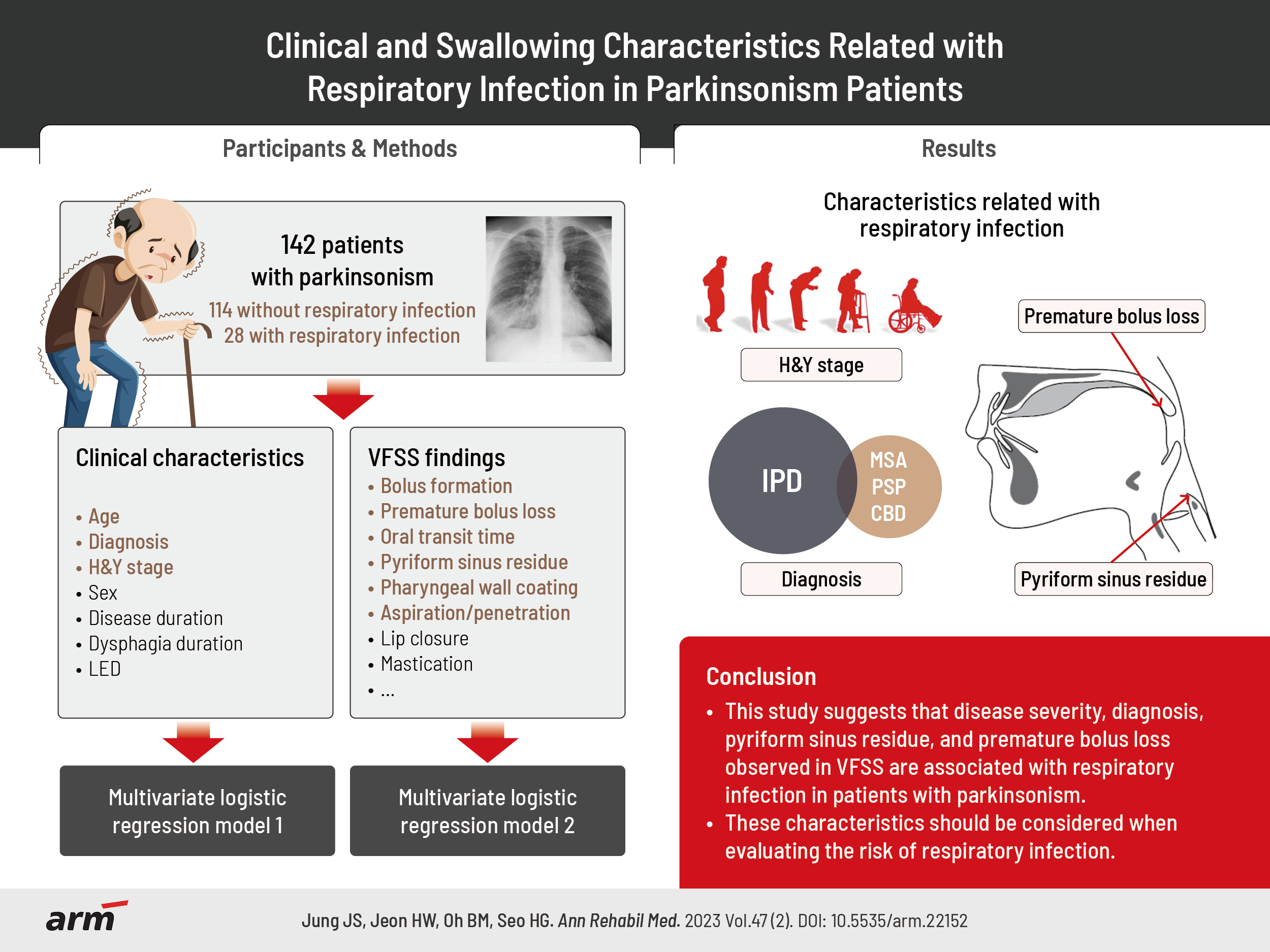
To investigate the clinical and swallowing characteristics related to respiratory infection in patients with parkinsonism.
Methods
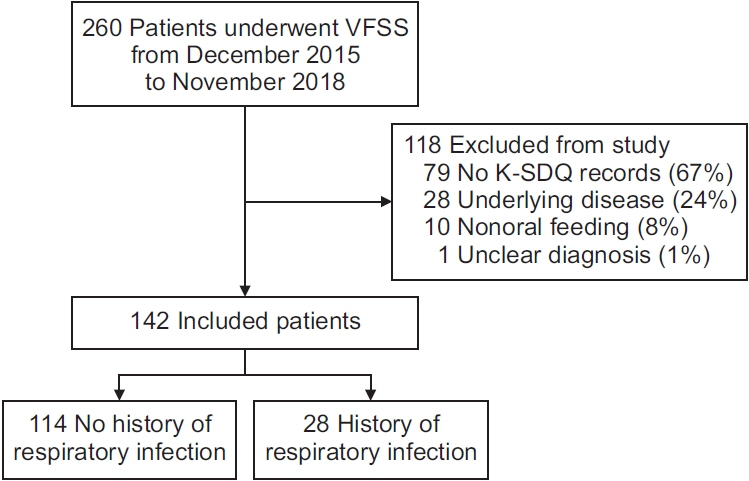
One hundred and forty-two patients with parkinsonism who underwent videofluoroscopic swallowing studies (VFSS) were enrolled in this study. The initial clinical and VFSS characteristics were compared between patients with and without a history of respiratory infection in the past year. A multivariate logistic regression model was applied to identify clinical and swallowing characteristics related to respiratory infections.
Results
Patients with respiratory infections were older (74.75±10.20 years vs. 70.70±8.83 years, p=0.037), had a higher Hoehn and Yahr (H&Y) stage (stage IV–V, 67.9% vs. 49.1%; p=0.047), and were more likely to have a diagnosis of idiopathic Parkinson’s disease (IPD) (67.9% vs. 41.2%, p=0.011) than those without respiratory infections. Among VFSS findings, bolus formation, premature bolus loss, oral transit time, pyriform sinus residues, pharyngeal wall coatings, and penetration/aspiration were significantly worse in patients with respiratory infections (p<0.05). Regarding clinical characteristics, higher H&Y stage (odds ratio [OR], 3.174; 95% confidence interval [CI], 1.226–8.216; p=0.017) and diagnosis of IPD (OR, 0.280, 95% CI, 0.111–0.706; p=0.007) were significantly related to respiratory infections in the multivariate analysis. Among VFSS findings, pyriform sinus residue (OR, 14.615; 95% CI, 2.257–94.623; p=0.005) and premature bolus loss (OR, 5.151; 95% CI, 1.047–25.338; p=0.044) were also significantly associated with respiratory infection.
Conclusion
This study suggests that disease severity, diagnosis, pyriform sinus residue, and premature bolus loss observed in VFSS are associated with respiratory infection in patients with parkinsonism.
Figure
Reference
-
1. Kwon M, Lee JH. Oro-pharyngeal dysphagia in Parkinson’s disease and related movement disorders. J Mov Disord. 2019; 12:152–60.
Article2. Litvan I. What is an atypical Parkinsonian disorder?. In : Litvan I, editor. Atypical Parkinsonian disorders: clinical and research aspects. Totowa: Humana Press;2005. p. 1–9.3. Coelho M, Marti MJ, Tolosa E, Ferreira JJ, Valldeoriola F, Rosa M, et al. Late-stage Parkinson’s disease: the Barcelona and Lisbon cohort. J Neurol. 2010; 257:1524–32.
Article4. Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, et al. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997; 99:106–12.
Article5. Kalf JG, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012; 18:311–5.
Article6. Litvan I. Parkinsonian features: when are they Parkinson disease? JAMA. 1998; 280:1654–5.7. Leopold NA, Daniels SK. Supranuclear control of swallowing. Dysphagia. 2010; 25:250–7.
Article8. Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013; 72:119–29.
Article9. Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, et al. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013; 72:614–23.
Article10. Morgante L, Salemi G, Meneghini F, Di Rosa AE, Epifanio A, Grigoletto F, et al. Parkinson disease survival: a population-based study. Arch Neurol. 2000; 57:507–12.11. Won JH, Byun SJ, Oh BM, Park SJ, Seo HG. Risk and mortality of aspiration pneumonia in Parkinson’s disease: a nationwide database study. Sci Rep. 2021; 11:6597.
Article12. Won JH, Byun SJ, Oh BM, Kim HJ, Park SJ, Seo HG. Pneumonia risk and its associated factors in Parkinson’s disease: a national database study. J Neurol Sci. 2020; 415:116949.
Article13. Chang YP, Yang CJ, Hu KF, Chao AC, Chang YH, Hsieh KP, et al. Risk factors for pneumonia among patients with Parkinson’s disease: a Taiwan nationwide population-based study. Neuropsychiatr Dis Treat. 2016; 12:1037–46.14. Costa MM. Videofluoroscopy: the gold standard exam for studying swallowing and its dysfunction. Arq Gastroenterol. 2010; 47:327–8.
Article15. Argolo N, Sampaio M, Pinho P, Melo A, Nóbrega AC. Videofluoroscopic predictors of penetrationaspiration in Parkinson’s disease patients. Dysphagia. 2015; 30:751–8.
Article16. Lee HH, Seo HG, Kim KD, Lee SH, Lee WH, Oh BM, et al. Characteristics of early oropharyngeal dysphagia in patients with multiple system atrophy. Neurodegener Dis. 2018; 18:84–90.
Article17. Clark HM, Stierwalt JAG, Tosakulwong N, Botha H, Ali F, Whitwell JL, et al. Dysphagia in progressive supranuclear palsy. Dysphagia. 2020; 35:667–76.
Article18. Robbins J, Gensler G, Hind J, Logemann JA, Lindblad AS, Brandt D, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008; 148:509–18. Erratum in: Ann Intern Med 2008;148:715.
Article19. Tomita S, Oeda T, Umemura A, Kohsaka M, Park K, Yamamoto K, et al. Video-fluoroscopic swallowing study scale for predicting aspiration pneumonia in Parkinson’s disease. PLoS One. 2018; 13:e0197608.
Article20. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55:181–4.
Article21. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008; 71:670–6.
Article22. Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDSSPSP international workshop. Neurology. 1996; 47:1–9.
Article23. McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005; 65:1863–72. Erratum in: Neurology 2005;65:1992.
Article24. Lee SY, Cheon SM, Kim JW. Validation of Korean version of swallowing disturbance questionnaire and characteristics of patients with dysphagia in Parkinson’s disease. Paper presented at: 2016 Korean Movement Disorder Society Spring Symposium; 2016 Apr 8-9; Seoul, Korea.25. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010; 25:2649–53.
Article26. Kim DH, Choi KH, Kim HM, Koo JH, Kim BR, Kim TW, et al. Inter-rater reliability of Videofluoroscopic Dysphagia Scale. Ann Rehabil Med. 2012; 36:791–6.
Article27. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996; 11:93–8.
Article28. Kim J, Oh BM, Kim JY, Lee GJ, Lee SA, Han TR. Validation of the videofluoroscopic dysphagia scale in various etiologies. Dysphagia. 2014; 29:438–43.
Article29. Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov Disord. 2007; 22:1917–21.
Article30. Cohen JT, Manor Y. Swallowing disturbance questionnaire for detecting dysphagia. Laryngoscope. 2011; 121:1383–7.
Article31. Hobson P, Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson’s disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open. 2018; 8:e018969.
Article32. Fall PA, Saleh A, Fredrickson M, Olsson JE, Granérus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov Disord. 2003; 18:1312–6.
Article33. Müller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, et al. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol. 2001; 58:259–64.
Article34. Wintzen AR, Badrising UA, Roos RA, Vielvoye J, Liauw L, Pauwels EK. Dysphagia in ambulant patients with Parkinson’s disease: common, not dangerous. Can J Neurol Sci. 1994; 21:53–6.
Article35. Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dysphagia. 2016; 31:24–32.
Article36. Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson’s disease. Dysphagia. 1998; 13:95–100.
Article37. Van Lieshout PH, Steele CM, Lang AE. Tongue control for swallowing in Parkinson’s disease: effects of age, rate, and stimulus consistency. Mov Disord. 2011; 26:1725–9.
Article38. Gandhi P, Steele CM. Effectiveness of interventions for dysphagia in Parkinson disease: a systematic review. Am J Speech Lang Pathol. 2022; 31:463–85.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Analysis of Blepharospasm and Apraxia of Eyelid Opening in Patients with Parkinsonism
- Bipolar Hemiarthroplasty of Displaced Femoral Neck Fractures in Pakinsonism Patients
- Clinical Evaluation of the Swallowing
- Relationship Between Swallowing Function and Maximum Phonation Time in Patients With Parkinsonism
- Instrumental Assessment of Swallowing