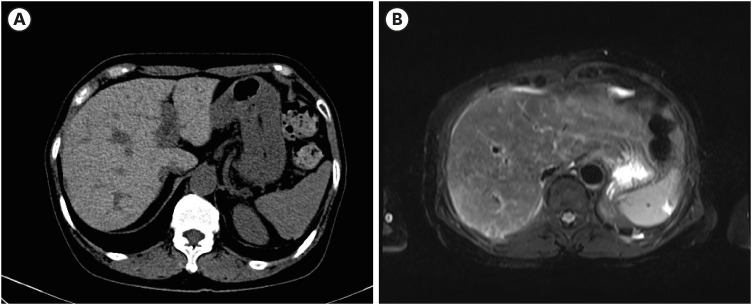
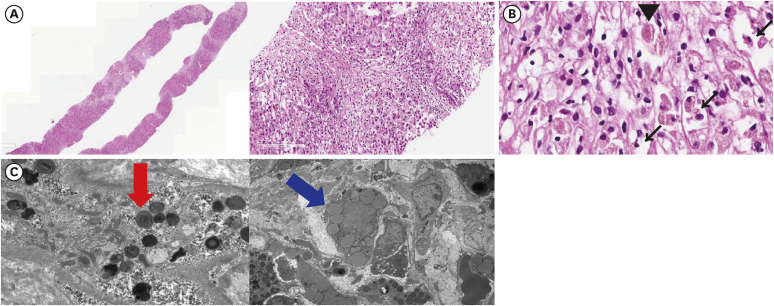
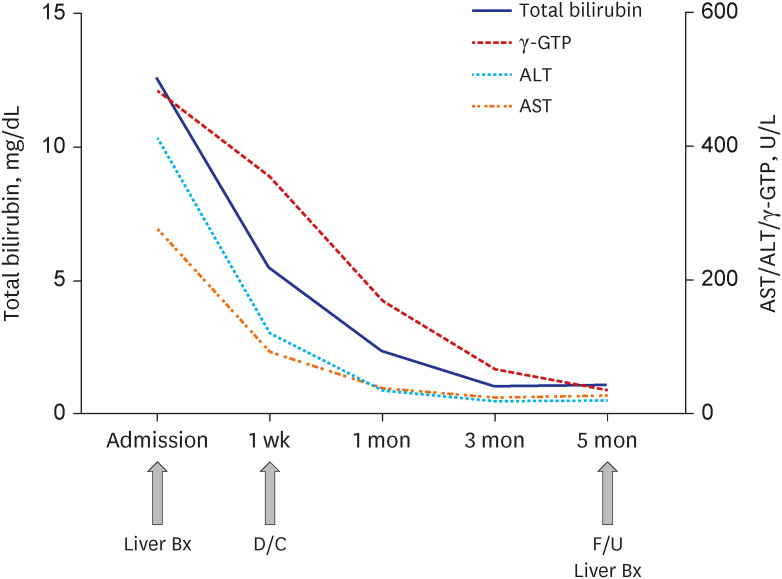
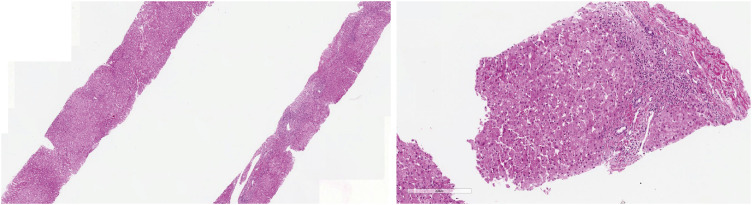
J Korean Med Sci.
2023 Apr;38(14):e105. 10.3346/jkms.2023.38.e105.
Case 9: A 62-Year-Old Woman With Jaundice and General Weakness
- Affiliations
-
- 1The Catholic University Liver Research Center, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Hepatology, Department of Internal Medicine, College of Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 3Division of Hepatology, Department of Internal Medicine, College of Medicine, Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 4Division of Hepatology, Department of Internal Medicine, College of Medicine, Eunpyeong St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- KMID: 2541545
- DOI: http://doi.org/10.3346/jkms.2023.38.e105
Figure
Reference
-
1. Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008; 48(1):169–176. PMID: 18537184.
Article2. Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol. 2021; 27(1):58–69. PMID: 33291862.
Article3. Lee JY, Yoo KH, Hahn SH. HFE gene mutation, C282Y causing hereditary hemochromatosis in Caucasian is extremely rare in Korean population. J Korean Med Sci. 2000; 15(2):179–182. PMID: 10803694.
Article4. Bacon BR, Adams PC, Kowdley KV, Powell LW, Tavill AS. American Association for the Study of Liver Diseases. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011; 54(1):328–343. PMID: 21452290.
Article5. Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, et al. Ferritin - from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun. 2022; 126:102778. PMID: 34883281.6. Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023; 77(3):1036–1065. PMID: 35899384.7. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases;2012.8. Low EX, Zheng Q, Chan E, Lim SG. Drug induced liver injury: East versus West - a systematic review and meta-analysis. Clin Mol Hepatol. 2020; 26(2):142–154. PMID: 31816676.
Article9. Lee HA, Chang Y, Sung PS, Yoon EL, Lee HW, Yoo JJ, et al. Therapeutic mechanisms and beneficial effects of non-antidiabetic drugs in chronic liver diseases. Clin Mol Hepatol. 2022; 28(3):425–472. PMID: 35850495.
Article10. Buggey J, Kappus M, Lagoo AS, Brady CW. Amiodarone-induced liver injury and cirrhosis. ACG Case Rep J. 2015; 2(2):116–118. PMID: 26157932.
Article11. Gregory SA, Webster JB, Chapman GD. Acute hepatitis induced by parenteral amiodarone. Am J Med. 2002; 113(3):254–255. PMID: 12208392.
Article12. Gayam V, Khalid M, Dahal S, Garlapati P, Gill A, Alex R, et al. Fatal acute liver failure with intravenous amiodarone: a case report and literature review. Gastroenterol Res. 2018; 11(1):62–63.
Article13. Jaiswal P, Attar BM, Yap JE, Devani K, Jaiswal R, Wang Y, et al. Acute liver failure with amiodarone infusion: a case report and systematic review. J Clin Pharm Ther. 2018; 43(1):129–133. PMID: 28714083.14. Sung PS, Yoon SK. Amiodarone hepatotoxicity. Hepatology. 2012; 55(1):325–326. PMID: 21898482.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Hyperkalemia discovered immediately after the Induction of General Anesthesia
- A Case of Obstructive Jaundice Caused by Extrinsic Compresson of Biliary Cystadenoma of the Common Hepatic Duct
- A Case of Acute Fatty Liver of Pregnancy
- A Case of Leiomyoma in the Common Bile Duct
- Ultrasonography of jaundice