Ann Pediatr Endocrinol Metab.
2023 Mar;28(1):34-41. 10.6065/apem.2244026.013.
Factors affecting bone mineral density in children and adolescents with secondary osteoporosis
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2540785
- DOI: http://doi.org/10.6065/apem.2244026.013
Abstract
- Purpose
This study aimed to investigate the clinical factors associated with bone mineral density (BMD) among children and adolescents with osteoporosis secondary to treatment for underlying clinical conditions.
Methods
We retrospectively reviewed the medical records of patients aged 10–18 years and evaluated them for lumbar spine BMD (LSBMD) after treatment for underlying diseases, including hemato-oncologic, rheumatologic system, and inflammator y bowel diseases. LSBMD measured by dual-energy x-ray absorptiometry (DXA) performed from March 2019 to March 2021 was evaluated. We analyzed 117 patients who underwent initial DXA after treatment for underlying diseases.
Results
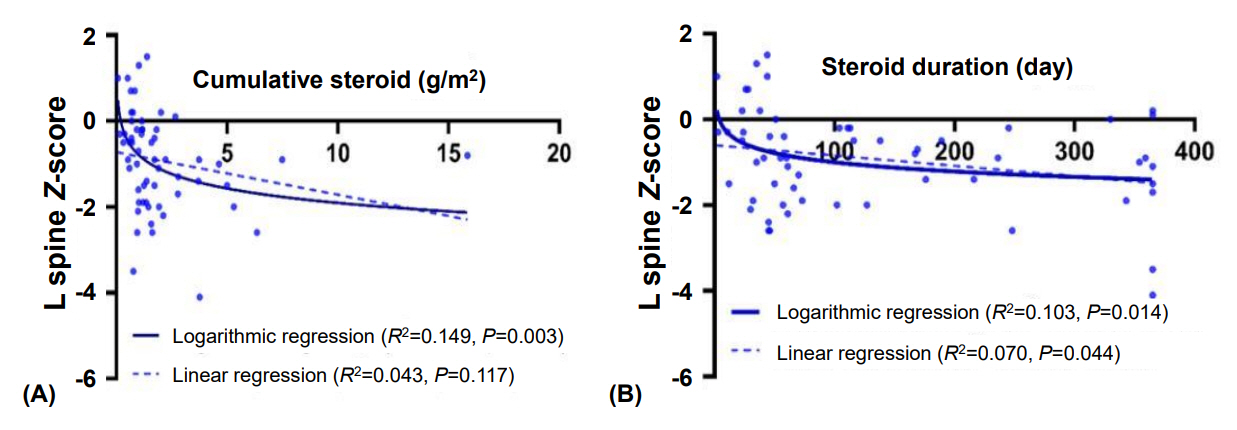
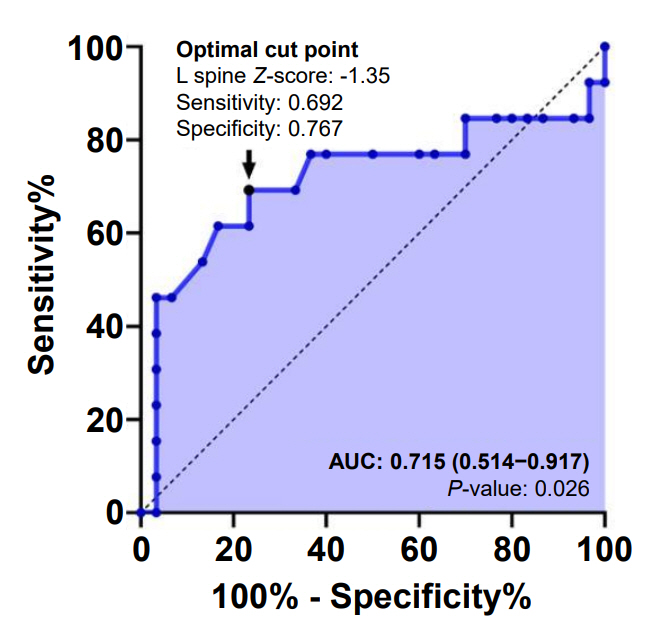
Subjects in this study had multiple underlying diseases: hemato-oncologic (78.6%), rheumatologic (11.1%), and inflammatory bowel diseases (10.3%). There was no significant association between the z-score and bone metabolic markers (P>0.05). However, higher cumulative glucocorticoid (GC) dose significantly reduced LSBMD z-score (P=0.029). Moreover, the association between cumulative dose of GC and initial z-score of LSBMD was significant in logarithmic regression analysis (P=0.003, R2=0.149). GC accumulation was a significant risk factor for vertebral fracture when the initial BMD was evaluated after treatment (P=0.043). Bone metabolic markers did not significantly influence the risk of vertebral fracture.
Conclusion
Initial bone mass density of the lumbar spine evaluated after long-term GC use for underlying diseases is a predictor of further vertebral fractures.
Figure
Reference
-
References
1. Di Iorgi N, Maruca K, Patti G, Mora S. Update on bone density measurements and their interpretation in children and adolescents. Best Pract Res Clin Endocrinol Metab. 2018; 32:477–98.2. Ahn MB, Suh BK. Bone morbidity in pediatric acute lymphoblastic leukemia. Ann Pediatr Endocrinol Metab. 2020; 25:1–9.3. Steffey CL. Pediatric osteoporosis. Pediatr Rev. 2019; 40:259–61.4. Bachrach LK, Gordon CM; SECTION ON ENDOCRINOLOGY. Bone densitometry in children and adolescents. Pediatrics. 2016; 138:e20162398.5. Pezzuti IL, Kakehasi AM, Filgueiras MT, de Guimaraes JA, de Lacerda IAC, Silva IN. Imaging methods for bone mass evaluation during childhood and adolescence: an update. J Pediatr Endocrinol Metab. 2017; 30:485–97.6. Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive summary of the 2019 ISCD Position Development Conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019; 22:453–71.7. Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions. J Clin Densitom. 2014; 17:275–80.8. Grover M, Bachrach LK. Osteoporosis in children with chronic illnesses: diagnosis, monitoring, and treatment. Curr Osteoporos Rep. 2017; 15:271–82.9. Nobile S, Grand RJ, Pappa HM. Risk factors for low bone mineral density in pediatric inflammatory bowel disease: the positive role of physical activity. Eur J Gastroenterol Hepatol. 2018; 30:471–6.10. Yi KH, Hwang JS, Kim EY, Lee JA, Kim DH, Lim JS. Reference values for bone mineral density according to age with body size adjustment in Korean children and adolescents. J Bone Miner Metab. 2014; 32:281–9.11. Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1996; 11:984–96.12. LeBlanc CM, Ma J, Taljaard M, Roth J, Scuccimarri R, Miettunen P, et al. Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders. J Bone Miner Res. 2015; 30:1667–75.13. Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, et al. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res. 2018; 33:1435–43.14. Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams textbook of endocrinology. 13 ed. Philadelphia (PA): Saunders;2016.15. Kang MJ, Lim JS. Bone mineral density deficits in childhood cancer survivors: pathophysiology, prevalence, screening, and management. Korean J Pediatr. 2013; 56:60–7.16. Jayasena A, Atapattu N, Lekamwasam S. Treatment of glucocorticoid-induced low bone mineral density in children: a systematic review. Int J Rheum Dis. 2015; 18:287–93.17. Ward LM. Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol (Lausanne). 2020; 11:576.18. Aljebab F, Choonara I, Conroy S. Systematic review of the toxicity of long-course oral corticosteroids in children. PLoS One. 2017; 12:e0170259.19. Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. 2014; 44:47–54.20. Lee HJ, Song Bs, Kim DH, Kim SY, Cho JB, Kim DH, et al. Bone mineral density reference of 10-20 year-old korean children and adolescents - based on hologic DXA from the Korean National Health and Nutrition Examination Surveys. J Korean Soc Pediatr Endocrinol. 2011; 16:92–9.21. Kim A, Baek S, Park S, Shin J. Bone mineral density of femur and lumbar and the relation between fat mass and lean mass of adolescents: based on Korea National Health and Nutrition Examination Survey (KNHNES) from 2008 to 2011. Int J Environ Res Public Health. 2020; 17:4471.22. Weber DR, Boyce A, Gordon C, Hogler W, Kecskemethy HH, Misra M, et al. The utility of DXA assessment at the forearm, proximal femur, and lateral distal femur, and vertebral fracture assessment in the pediatric population: 2019 ISCD Official Position. J Clin Densitom. 2019; 22:567–89.23. Singh DN, Krishnamurthy S, Kamalanathan SK, Harichandrakumar KT, Sivamurukan P. Three-monthly bolus vitamin D supplements (1000 vs 400 IU/day) for prevention of bone loss in children with difficult-to-treat nephrotic syndrome: a randomised clinical trial. Paediatr Int Child Health. 2018; 38:251–60.24. Galindo-Zavala R, Bou-Torrent R, Magallares-Lopez B, Mir-Perello C, Palmou-Fontana N, Sevilla-Perez B, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. 2020; 18:20.25. Rooney M, Bishop N, Davidson J, Beresford MW, Pilkington C, Donagh JM, et al. The prevention and treatment of glucocorticoid-induced osteopaenia in juvenile rheumatic disease: a randomised double-blind controlled trial. EClinicalMedicine. 2019; 12:79–87.26. Ward L, Tricco AC, Phuong P, Cranney A, Barrowman N, Gaboury I, et al. Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev. 2007; 2007:CD005324.27. Simm PJ, Biggin A, Zacharin MR, Rodda CP, Tham E, Siafarikas A, et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J Paediatr Child Health. 2018; 54:223–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Osteoporosis
- Pediatric dual-energy X-ray absorptiometry: interpretation and clinical and research application
- A Study of Factors Affecting Bone Mineral Density in Children : Anthropometric Measurements, Socioeconomic Factors, Family History, and Other Environmental Factors
- Change of Bone Mineral Density after One-year Alendronate Treatment in Premenopausal Women with Low Bone Density
- Diagnosis and Management of Osteoporosis in Children and Adolescents