J Korean Med Sci.
2023 Mar;38(11):e94. 10.3346/jkms.2023.38.e94.
Relationship Between Coronavirus Disease 2019 Vaccination Rates and Rare But Potentially Fatal Adverse Events: A Regression Discontinuity Analysis of Western Countries
- Affiliations
-
- 1Department of Politics and IR, University of Oxford, Oxford, United Kingdom
- 2Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Artificial Intelligence and Big-Data Convergence Center, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 4Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- KMID: 2540655
- DOI: http://doi.org/10.3346/jkms.2023.38.e94
Abstract
- Background
Owing to limited experience with the new vaccine platforms, discussion of vaccine safety is inevitable. However, media coverage of adverse events of special interest could influence the vaccination rate; thus, evaluating the outcomes of adverse events of special interest influencing vaccine administration is crucial.
Methods
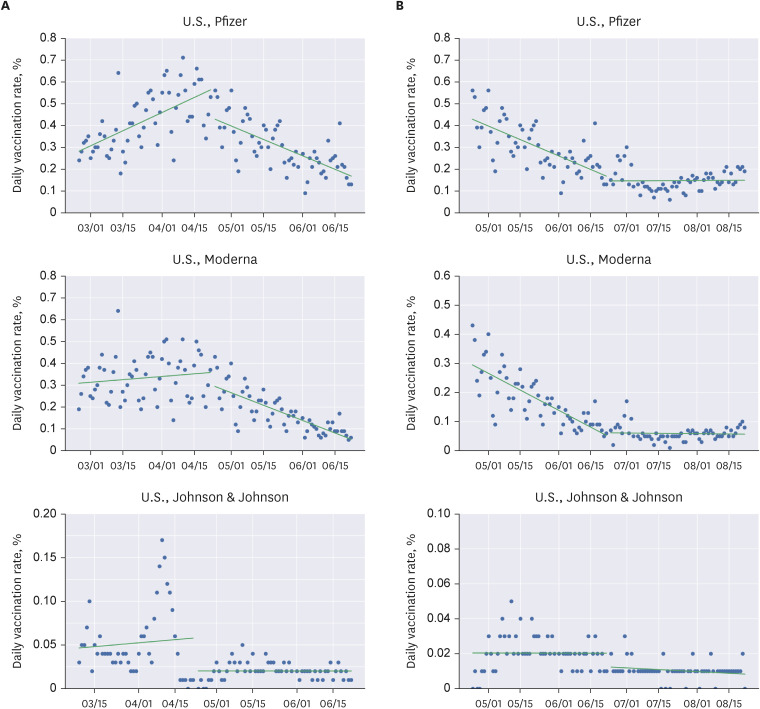
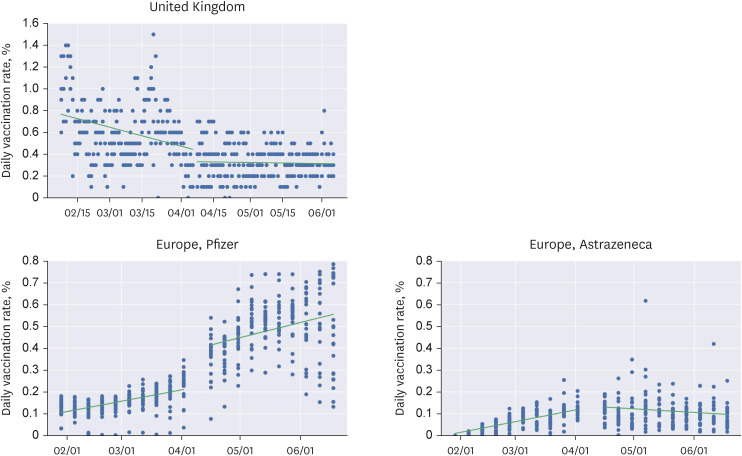
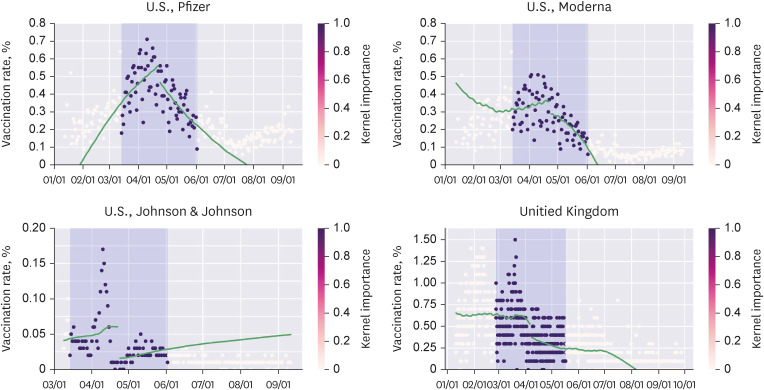
We conducted regression discontinuity in time analysis to calculate the local average treatment effect (LATE) using datasets from Our World in Data and Johns Hopkins University Center for Systems Science and Engineering. For the United States, the United Kingdom, and Europe, the cutoff points were April 23rd and June 23rd, April 7th, and the 14th week of 2021, respectively.
Results
The LATE of the Advisory Committee on Immunization Practices (ACIP) meeting held on April 23rd was −0.249 for all vaccines, −0.133 (−0.189 to −0.076) for Pfizer, −0.064 (−0.115 to −0.012) for Moderna, and −0.038 (−0.047 to −0.030) for Johnson & Johnson. Discontinuities were observed for all three types of vaccines in the United States. The June 23rd meeting of the ACIP (mRNA vaccines and myocarditis) did not convene any discontinuities. Furthermore, there was no significant drop in the weekly average vaccination rates in Europe following the European Medicines Agency (EMA) statement on April 7th. Conversely, there was a significant drop in the first-dose vaccination rates in the United Kingdom related to the EMA report. The first-dose vaccination rate for all vaccines changed by −0.104 (−0.176 to −0.032).
Conclusion
Although monitoring and reporting of adverse events of special interest are important, a careful approach towards public announcements is warranted.
Figure
Reference
-
1. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021; 385(7):585–594. PMID: 34289274.2. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021; 326(14):1390–1399. PMID: 34477808.3. World Health Organization (WHO). COVID-19 advice for the public: getting vaccinated. Updated July 14, 2021. Accessed October 5, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice .4. McGill COVID19 Vaccine Tracker Team. Vaccines candidates by trial phase. Updated October 1, 2021. Accessed October 5, 2021. https://covid19.trackvaccines.org/vaccines/?fbclid=IwAR0rJdUx1T1j9TalYCJzwvnqm6MWluN2wwWtnuDP0GW6YvKLh3nW-Ex0ZKU .5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.6. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.7. Østergaard SD, Schmidt M, Horváth-Puhó E, Thomsen RW, Sørensen HT. Thromboembolism and the Oxford-AstraZeneca COVID-19 vaccine: side-effect or coincidence? Lancet. 2021; 397(10283):1441–1443. PMID: 33798498.8. Huh K, Na Y, Kim YE, Radnaabaatar M, Peck KR, Jung J. Predicted and observed incidence of thromboembolic events among Koreans vaccinated with ChAdOx1 nCoV-19 vaccine. J Korean Med Sci. 2021; 36(27):e197. PMID: 34254476.9. European Medicines Agency (EMA). AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. Updated 2021. Accessed October 5, 2021. https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood .10. Wise J. Covid-19: how AstraZeneca lost the vaccine PR war. BMJ. 2021; 373(921):n921. PMID: 33853827.11. Dyer O. Covid-19: EMA defends AstraZeneca vaccine as Germany and Canada halt rollouts. BMJ. 2021; 373(883):n883. PMID: 33795216.12. Lee E, Lee YK, Kim TE, Hwang I, Jung YH, Lee HR, et al. Reports of anaphylaxis after coronavirus disease 2019 vaccination, South Korea, 26 February to 30 April 2021. Euro Surveill. 2021; 26(33):2100694. PMID: 34414880.13. MacNeil JR, Su JR, Broder KR, Guh AY, Gargano JW, Wallace M, et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients - United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(17):651–656. PMID: 33914723.14. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(27):977–982. PMID: 34237049.15. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021; 144(6):471–484. PMID: 34281357.16. World Health Organization (WHO). COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated guidance regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. Updated July 9, 2021. Accessed October 05, 2021. https://www.who.int/news/item/09-07-2021-gacvs-guidance-myocarditis-pericarditis-covid-19-mrna-vaccines .17. Pullan S, Dey M. Vaccine hesitancy and anti-vaccination in the time of COVID-19: a Google Trends analysis. Vaccine. 2021; 39(14):1877–1881. PMID: 33715904.18. Anderson ML. Subways, strikes, and slowdowns: the impacts of public transit on traffic congestion. Am Econ Rev. 2014; 104(9):2763–2796.19. Bakolis I, Stewart R, Baldwin D, Beenstock J, Bibby P, Broadbent M, et al. Changes in daily mental health service use and mortality at the commencement and lifting of COVID-19 ‘lockdown’ policy in 10 UK sites: a regression discontinuity in time design. BMJ Open. 2021; 11(5):e049721.20. Calonico S, Cattaneo MD, Titiunik R. Robust nonparametric confidence intervals for regression-discontinuity designs. Econometrica. 2014; 82(6):2295–2326.21. Park YS. Thrombosis and severe acute respiratory syndrome coronavirus 2 vaccines: vaccine-induced immune thrombotic thrombocytopenia. Clin Exp Pediatr. 2021; 64(8):400–405. PMID: 34237213.22. Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021; 6(10):1115–1117. PMID: 34185047.23. Smith M. Perceived safety drops substantially in France, Germany, Spain and Italy. Updated March 22, 2021. Accessed October 7, 2021. https://yougov.co.uk/topics/international/articles-reports/2021/03/22/europeans-now-see-astrazeneca-vaccine-unsafe-follo .24. Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, et al. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021; 20(10):1201–1209. PMID: 34488546.25. Karron RA, Key NS, Sharfstein JM. Assessing a rare and serious adverse event following administration of the Ad26.COV2.S vaccine. JAMA. 2021; 325(24):2445–2447. PMID: 33929484.26. Torjesen I. Covid-19 vaccine shortages: what is the cause and what are the implications? BMJ. 2021; 372(781):n781. PMID: 33741547.27. Beaubien J. It’s the vaccine that’s lost a lot of trust. But AstraZeneca still has its fans. Updated June 1, 2021. Accessed October 5, 2021. https://www.npr.org/sections/goatsandsoda/2021/06/01/1002067808/astrozenecas-rocky-rollout-the-woes-of-the-vaccine-of-the-world .28. Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino C, Hasell J, et al. Coronavirus Pandemic (COVID-19). Updated 2020. Accessed October 5, 2021. https://ourworldindata.org/coronavirus .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse events following vaccination against coronavirus disease 2019
- Post-vaccination COVID-19 deaths: a review of available evidence and recommendations for the global population
- Healthcare Systems and COVID-19 Mortality in Selected OECD Countries: A Panel Quantile Regression Analysis
- Vestibular Neuritis after COVID-19 Vaccination
- Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System