J Korean Med Sci.
2021 May;36(17):e114. 10.3346/jkms.2021.36.e114.
Adverse Events Following Immunization Associated with Coronavirus Disease 2019 Vaccination Reported in the Mobile Vaccine Adverse Events Reporting System
- Affiliations
-
- 1Division of Infectious Diseases, Department of Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Division of Pulmonary and Critical Care Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 3Department of Pediatrics, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2515805
- DOI: http://doi.org/10.3346/jkms.2021.36.e114
Abstract
- Background
Vaccination against coronavirus disease 2019 (COVID-19) is underway globally to prevent the infection caused by the severe acute respiratory syndrome coronavirus 2. We aimed to investigate the adverse events following immunization (AEFIs) for COVID-19 among healthcare workers (HCWs).
Methods
This was a retrospective study of the AEFIs associated with the first dose of the ChAdOx1 nCoV-19 vaccine at the Kosin University Gospel Hospital from March 3 to March 22, 2021. We investigated the systemic and local adverse events during the 7 days following the vaccination using the Mobile Vaccine Adverse Events Reporting System (MVAERS) developed by our hospital.
Results
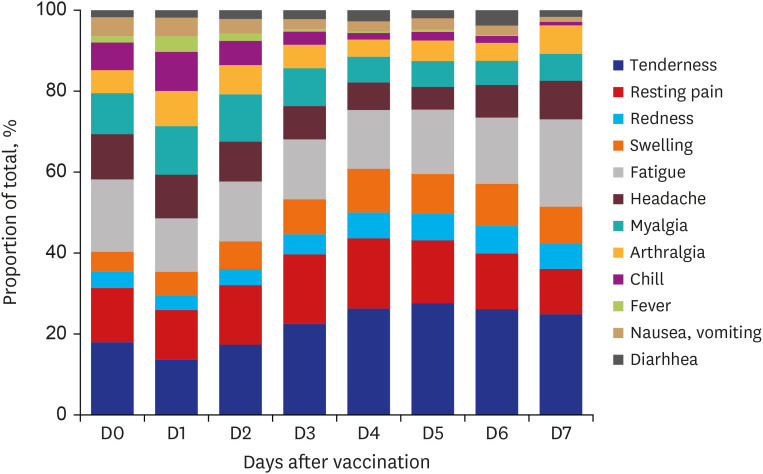
A total of 1,503 HCWs were vaccinated, and the data of 994 HCWs were reported in the MVAERS. The most commonly reported AEFIs were tenderness at the injection site (94.5%), fatigue (92.9%), pain at the injection site (88.0%), and malaise (83.8%). The severity of most AEFIs was mild-to-moderate, and the severity and number of AEFIs were less in the older age group. There were no serious events requiring hospitalization, and most AEFIs improved within a few days.
Conclusion
The AEFIs associated with the ChAdOx1 nCoV-19 vaccine were tolerable, and the use of the MVAERS was helpful in monitoring the AEFIs. The use of MVAERS will help in sharing accurate and ample information about vaccination against COVID-19.
Figure
Cited by 3 articles
-
Audiovisual Files for Expanding the
Journal of Korean Medical Science Content Outreach
Sung-Tae Hong
J Korean Med Sci. 2021;36(20):e154. doi: 10.3346/jkms.2021.36.e154.Emergency Department Utilization by In-hospital Healthcare Workers after COVID-19 Vaccination
Min Ji Park, Yoo Jin Choi, Sangchun Choi
J Korean Med Sci. 2021;36(27):e196. doi: 10.3346/jkms.2021.36.e196.Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19
Seong-Heon Wie, Jaehun Jung, Woo Joo Kim
J Korean Med Sci. 2023;38(44):e371. doi: 10.3346/jkms.2023.38.e371.
Reference
-
1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2021. Accessed March 23, 2021. https://covid19.who.int.2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article3. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.4. CDC COVID-19 Response Team. Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(2):46–51. PMID: 33444297.5. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. Forthcoming. 2021.
Article6. Farooq F, Rathore FA. COVID-19 vaccination and the challenge of infodemic and disinformation. J Korean Med Sci. 2021; 36(10):e78. PMID: 33724740.
Article7. U.S. Food & Drug Administration. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Updated 2007. Accessed March 26, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical.8. European Medicines Agency. Assessment report COVID-19 vaccine AstraZeneca. Updated 2021. Accessed March 26, 2021. https://www.ema.europa.eu/en/documents/assessment-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-public-assessment-report_en.pdf.9. Huh K, Kim YE, Radnaabaatar M, Lee DH, Kim DW, Shin SA, et al. Estimating baseline incidence of conditions potentially associated with vaccine adverse events: a call for surveillance system using the Korean National Health Insurance Claims Data. J Korean Med Sci. 2021; 36(9):e67. PMID: 33686812.
Article10. Korea Centers for Disease Control and Prevention. COVID-19 domestic occurrence and vaccination status. Updated 2021. Accessed March 26, 2021. http://www.cdc.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=712838&cg_code=&act=view&nPage=1.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 vaccine safety monitoring in the Republic of Korea: February 26, 2021 to April 30, 2021
- Adverse events of the Pfizer-BioNTech COVID-19 vaccine in Korean children and adolescents aged 5 to 17 years
- COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021
- Safety monitoring of COVID-19 vaccination among adolescents aged 12 to 17 years old in the Republic of Korea
- Self-reported adverse events after 2 doses of COVID-19 vaccine in Korea