Anesth Pain Med.
2023 Jan;18(1):29-36. 10.17085/apm.22201.
Effect of neoadjuvant chemotherapy on effect-site concentration of propofol for sedation in patients with breast cancer
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- KMID: 2540331
- DOI: http://doi.org/10.17085/apm.22201
Abstract
- Background
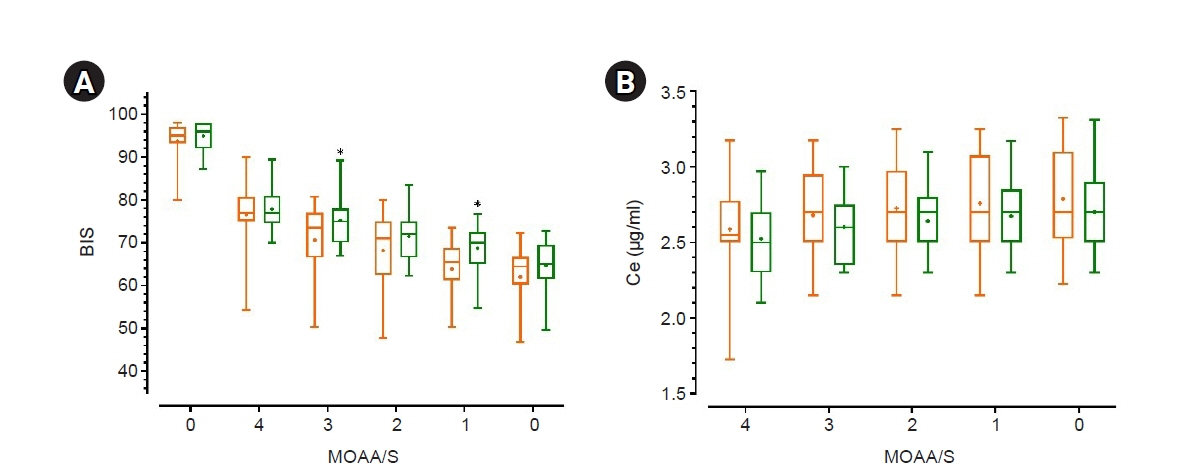
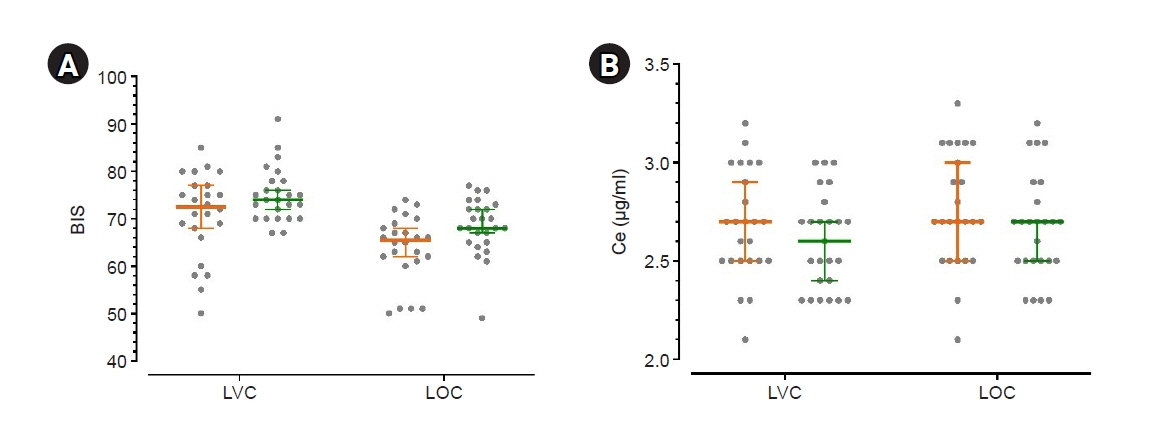
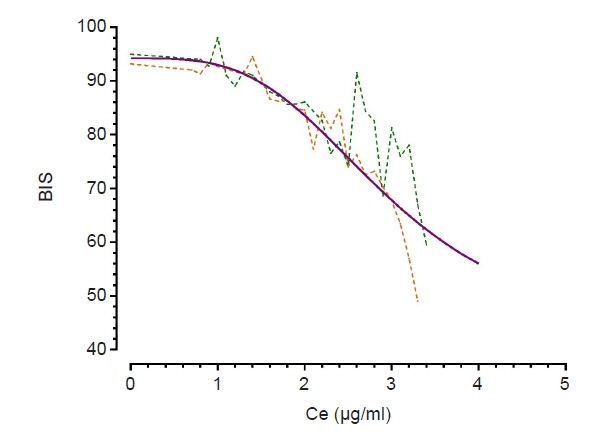
Some studies have demonstrated that chemotherapy drugs enhance sensitivity to anesthetics owing to its systemic toxicity, while others have demonstrated that chemotherapy drugs have no effect. This study aimed to determine whether neoadjuvant chemotherapy influences the effect-site concentration (Ce) of propofol for sedation in patients withbreast cancer.Methods: This study included patients aged 19–75 years who were scheduled to undergobreast cancer surgery under general anesthesia. Patients who received neoadjuvant chemotherapy were assigned to group C, whereas those who never received chemotherapy wereassigned to group N. Propofol was administered through an effect-site target-controlled infusion, and the Modified Observer’s Assessment of Alertness/Sedation scale (MOAA/S) scoreand Bispectral Index (BIS) were recorded. When the plasma concentration and Ce wereequal to the target Ce, and if the MOAA/S score did not change, the target Ce was increasedby 0.2 μg/ml; otherwise, the Ce was maintained for 2 min and then increased. This processwas repeated until the MOAA/S score became 0.Results: No significant differences were observed in Ce values at each sedation level between both groups. Ce values for loss of consciousness (LOC) of groups C and N were 2.76± 0.29 and 2.67 ± 0.27 μg/ml (P = 0.285), respectively. However, the BIS value at LOC ofgroup C (63.87 ± 7.04) was lower than that (68.44 ± 6.01) of group N (P = 0.018).Conclusions: Neoadjuvant chemotherapy for breast cancer has no effect on the Ce ofpropofol for sedation.
Figure
Reference
-
1. Sapunar F, Smith IE. Neoadjuvant chemotherapy for breast cancer. Ann Med. 2000; 32:43–50.
Article2. Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond). 2016; 12:480–91.
Article3. Shien T, Iwata H. Adjuvant and neoadjuvant therapy for breast cancer. Jpn J Clin Oncol. 2020; 50:225–9.
Article4. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet 2021; 397: 1750-69. Erratum in: Lancet. 2021; 397:1710.5. Fisusi FA, Akala EO. Drug combinations in breast cancer therapy. Pharm Nanotechnol. 2019; 7:3–23.
Article6. Gehdoo RP. Anticancer chemotherapy and it's anaesthetic implications (current concepts). Indian J Anaesth. 2009; 53:18–29.7. Zaniboni A, Prabhu S, Audisio RA. Chemotherapy and anaesthetic drugs: too little is known. Lancet Oncol. 2005; 6:176–81.
Article8. Taillibert S, Le Rhun E, Chamberlain MC. Chemotherapy-related neurotoxicity. Curr Neurol Neurosci Rep. 2016; 16:81.
Article9. Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009; 63:761–7.
Article10. Dutta V. Chemotherapy, neurotoxicity, and cognitive changes in breast cancer. J Cancer Res Ther. 2011; 7:264–9.11. Du W, Li C, Wang H, Zhao A, Shen J, Yong F, et al. Effect of neoadjuvant chemotherapy on sevoflurane MAC-BAR value of patients undergoing radical stomach carcinoma surgery. Int J Clin Exp Med. 2015; 8:5649–57.12. Wu G, Fu G, Zhang L, Zhang Z, Wang X. Effects of neoadjuvant chemotherapy on the depth of total intravenous anesthesia in patients with breast cancer undergoing unilateral modified radical mastectomy: a prospective observational study. Medicine (Baltimore). 2018; 97:e13776.13. Zhang L, Zuo M, Ma X, Dong Y. Effects of neoadjuvant chemotherapy on minimum alveolar concentration values of sevoflurane and desflurane in patients with hepatocellular carcinoma complicated with jaundice. Oncol Lett. 2018; 16:388–94.
Article14. He ZJ, Hu YH, Fan ZY. Median effective effect-site concentration of intravenous anesthetics for loss of consciousness in neoadjuvant chemotherapy patients. Chin Med J (Engl). 2011; 124:504–8.15. Ki S, Cho Y, Choi Y, Lim S, Kim M, Lee J. Effect of chemotherapy on effect-site concentration of propofol for loss of consciousness in patients with colorectal cancer. Korean J Anesthesiol. 2022; 75:160–7.
Article16. Schnider TW, Minto CF, Gambus PL, Andresen C, Goodale DB, Shafer SL, et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology. 1998; 88:1170–82.
Article17. Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990; 10:244–51.18. Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001; 28:231–52.19. Beal SL. Commentary on significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2002; 29:403–10; discussion 411-2.20. Kesler SR, Blayney DW. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016; 2:185–92.
Article21. Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007; 109:146–56.
Article22. Feng Y, Zhang XD, Zheng G, Zhang LJ. Chemotherapy-induced brain changes in breast cancer survivors: evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav. 2019; 13:1799–814.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Titration of Effect Site Concentration of Propofol for Conscious Sedation in Elderly Patients
- Correlations between the Modified Observer's Assessment of Alertness/Sedation Scale, Bispectral Index and Propofol Effect Site Concentrations in Sedated Elderly Patients under Regional Anesthesia
- Effect of chemotherapy on effect-site concentration of propofol for loss of consciousness in patients with colorectal cancer
- The Changes of Reaction Time to Visual and Auditory Stimulations during Propofol Administration for Conscious Sedation
- The Effects of Sedation Using Propofol on the Frequency of Retrograde Amnesia