Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Radiology, Korea University Guro Hospital, Seoul, Korea
- 2Department of Radiology, Yeouido St. Mary’s Hospital, College of Medicine, The Catholic University of Korea;
- 3Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Radiology, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 5Department of Radiology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- KMID: 2539825
- DOI: http://doi.org/10.3803/EnM.2023.1670
Abstract
- Background
This study investigated the diagnostic performance of biopsy criteria in four society ultrasonography risk stratification systems (RSSs) for thyroid nodules, including the 2021 Korean (K)-Thyroid Imaging Reporting and Data System (TIRADS).
Methods
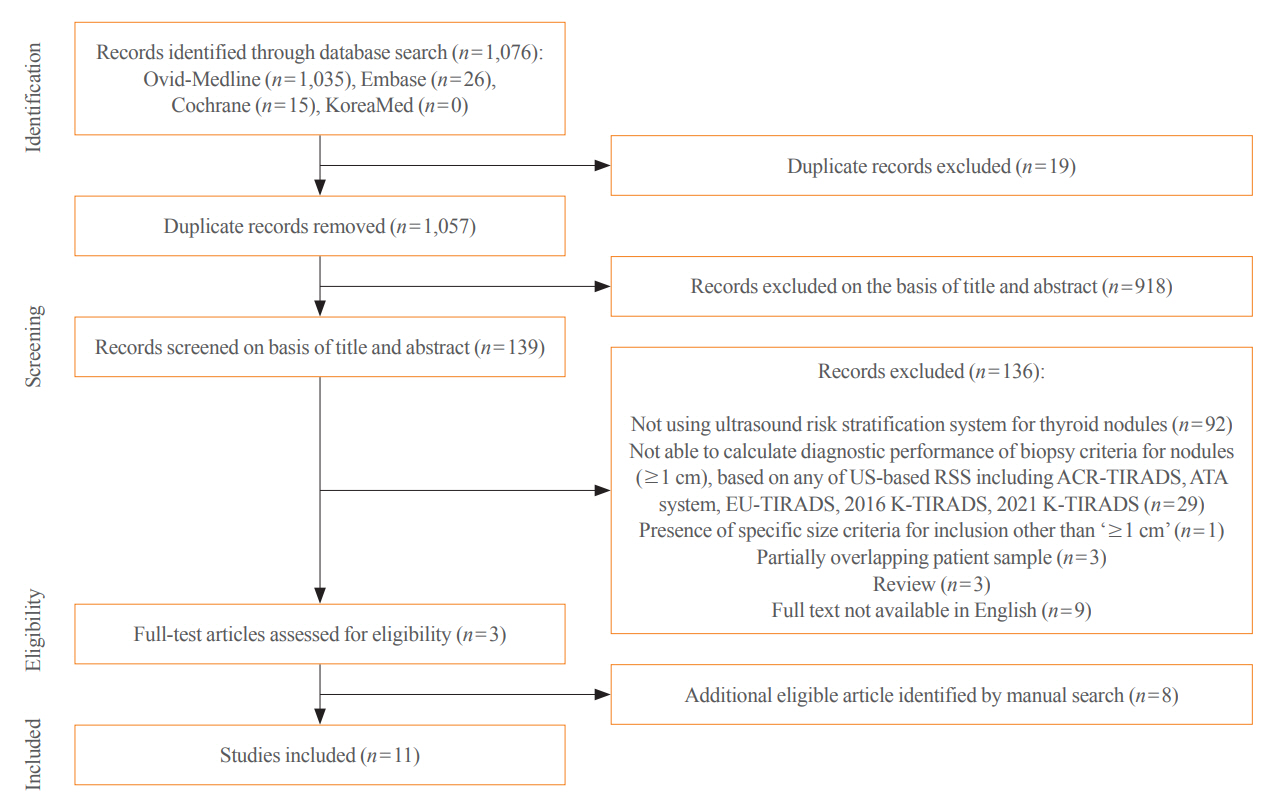
The Ovid-MEDLINE, Embase, Cochrane, and KoreaMed databases were searched and a manual search was conducted to identify original articles investigating the diagnostic performance of biopsy criteria for thyroid nodules (≥1 cm) in four widely used society RSSs.
Results
Eleven articles were included. The pooled sensitivity and specificity were 82% (95% confidence interval [CI], 74% to 87%) and 60% (95% CI, 52% to 67%) for the American College of Radiology (ACR)-TIRADS, 89% (95% CI, 85% to 93%) and 34% (95% CI, 26% to 42%) for the American Thyroid Association (ATA) system, 88% (95% CI, 81% to 92%) and 42% (95% CI, 22% to 67%) for the European (EU)-TIRADS, and 96% (95% CI, 94% to 97%) and 21% (95% CI, 17% to 25%) for the 2016 K-TIRADS. The sensitivity and specificity were 76% (95% CI, 74% to 79%) and 50% (95% CI, 49% to 52%) for the 2021 K-TIRADS1.5 (1.5-cm size cut-off for intermediate-suspicion nodules). The pooled unnecessary biopsy rates of the ACR-TIRADS, ATA system, EU-TIRADS, and 2016 K-TIRADS were 41% (95% CI, 32% to 49%), 65% (95% CI, 56% to 74%), 68% (95% CI, 60% to 75%), and 79% (95% CI, 74% to 83%), respectively. The unnecessary biopsy rate was 50% (95% CI, 47% to 53%) for the 2021 K-TIRADS1.5.
Conclusion
The unnecessary biopsy rate of the 2021 K-TIRADS1.5 was substantially lower than that of the 2016 K-TIRADS and comparable to that of the ACR-TIRADS. The 2021 K-TIRADS may help reduce potential harm due to unnecessary biopsies.
Figure
Cited by 5 articles
-
Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinol Metab. 2023;38(1):75-77. doi: 10.3803/EnM.2023.105.To Screen or Not to Screen?
Do Joon Park
Endocrinol Metab. 2023;38(1):69-71. doi: 10.3803/EnM.2023.104.The 2017 United States Preventive Services Task Force Recommendation for Thyroid Cancer Screening Is No Longer the Gold Standard
Ka Hee Yi
Endocrinol Metab. 2023;38(1):72-74. doi: 10.3803/EnM.2023.106.2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2023;16(1):1-31. doi: 10.11106/ijt.2023.16.1.1.Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules 2024
Young Joo Park, Eun Kyung Lee, Young Shin Song, Su Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2024;17(1):208-244. doi: 10.11106/ijt.2024.17.1.208.
Reference
-
1. Hoang JK, Grady AT, Nguyen XV. What to do with incidental thyroid nodules identified on imaging studies? Review of current evidence and recommendations. Curr Opin Oncol. 2015; 27:8–14.
Article2. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015; 25:1127–36.
Article3. Seib CD, Sosa JA. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin North Am. 2019; 48:23–35.
Article4. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81 Suppl 1:1–122.
Article5. Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract. 2016; 22:622–39.6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article7. Shin JH, Baek JH, Chung J, Ha EJ, Kim JH, Lee YH, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016; 17:370–95.
Article8. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017; 6:225–37.
Article9. Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017; 14:587–95.
Article10. Zhou J, Yin L, Wei X, Zhang S, Song Y, Luo B, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020; 70:256–79.11. Ha EJ, Chung SR, Na DG, Ahn HS, Chung J, Lee JY, et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2021; 22:2094–123.
Article12. Hayen A, Macaskill P, Irwig L, Bossuyt P. Appropriate statistical methods are required to assess diagnostic tests for replacement, add-on, and triage. J Clin Epidemiol. 2010; 63:883–91.
Article13. Kim DH, Chung SR, Choi SH, Kim KW. Accuracy of thyroid imaging reporting and data system category 4 or 5 for diagnosing malignancy: a systematic review and meta-analysis. Eur Radiol. 2020; 30:5611–24.
Article14. Kim PH, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Diagnostic performance of four ultrasound risk stratification systems: a systematic review and meta-analysis. Thyroid. 2020; 30:1159–68.
Article15. Ha EJ, Na DG, Baek JH, Sung JY, Kim JH, Kang SY. US fine-needle aspiration biopsy for thyroid malignancy: diagnostic performance of seven society guidelines applied to 2000 thyroid nodules. Radiology. 2018; 287:893–900.
Article16. Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J Clin Endocrinol Metab. 2019; 104:95–102.
Article17. Ha SM, Baek JH, Na DG, Suh CH, Chung SR, Choi YJ, et al. Diagnostic performance of practice guidelines for thyroid nodules: thyroid nodule size versus biopsy rates. Radiology. 2019; 291:92–9.
Article18. Xu T, Wu Y, Wu RX, Zhang YZ, Gu JY, Ye XH, et al. Validation and comparison of three newly-released thyroid imaging reporting and data systems for cancer risk determination. Endocrine. 2019; 64:299–307.
Article19. Yoon SJ, Na DG, Gwon HY, Paik W, Kim WJ, Song JS, et al. Similarities and differences between thyroid imaging reporting and data systems. AJR Am J Roentgenol. 2019; 213:W76–84.
Article20. Ha EJ, Na DG, Moon WJ, Lee YH, Choi N. Diagnostic performance of ultrasound-based risk-stratification systems for thyroid nodules: comparison of the 2015 American Thyroid Association guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology guidelines. Thyroid. 2018; 28:1532–7.
Article21. Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, et al. Comparison of performance characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association guidelines. AJR Am J Roentgenol. 2018; 210:1148–54.
Article22. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9.
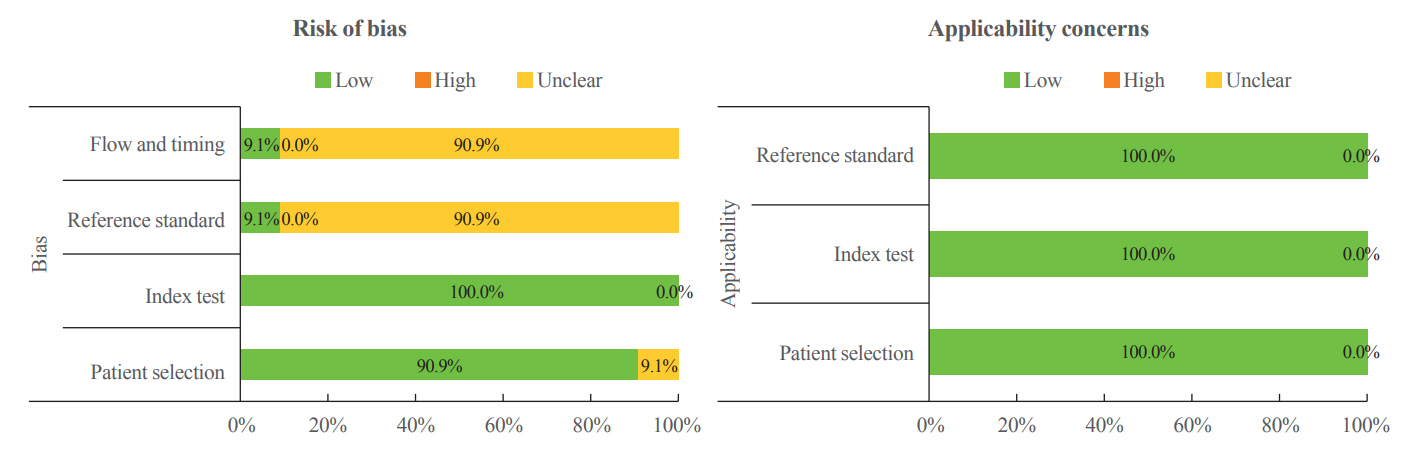
Article23. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–36.
Article24. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005; 58:882–93.
Article25. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article26. Kim KW, Lee J, Choi SH, Huh J, Park SH. Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers. Part I: General guidance and tips. Korean J Radiol. 2015; 16:1175–87.
Article27. Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002; 2:9.
Article28. Tan L, Tan YS, Tan S. Diagnostic accuracy and ability to reduce unnecessary FNAC: a comparison between four Thyroid Imaging Reporting Data System (TI-RADS) versions. Clin Imaging. 2020; 65:133–7.
Article29. Ha EJ, Shin JH, Na DG, Jung SL, Lee YH, Paik W, et al. Comparison of the diagnostic performance of the modified Korean Thyroid Imaging Reporting and Data System for thyroid malignancy with three international guidelines. Ultrasonography. 2021; 40:594–601.
Article30. Huh S, Yoon JH, Lee HS, Moon HJ, Park VY, Kwak JY. Comparison of diagnostic performance of the ACR and Kwak TIRADS applying the ACR TIRADS’ size thresholds for FNA. Eur Radiol. 2021; 31:5243–50.
Article31. Na DG, Paik W, Cha J, Gwon HY, Kim SY, Yoo RE. Diagnostic performance of the modified Korean Thyroid Imaging Reporting and Data System for thyroid malignancy according to nodule size: a comparison with five society guidelines. Ultrasonography. 2021; 40:474–85.
Article32. Eidt LB, Nunes de Oliveira C, Lagos YB, Solera GL, Izquierdo R, Meyer EL, et al. A prospective comparison of ACR-TIRADS and EU-TIRADS in thyroid nodule assessment for FNA-US. Clin Endocrinol (Oxf). 2023; 98:415–25.
Article33. Chung SR, Ahn HS, Choi YJ, Lee JY, Yoo RE, Lee YJ, et al. Diagnostic performance of the modified Korean thyroid imaging reporting and data system for thyroid malignancy: a multicenter validation study. Korean J Radiol. 2021; 22:1579–86.
Article34. Castellana M, Castellana C, Treglia G, Giorgino F, Giovanella L, Russ G, et al. Performance of five ultrasound risk stratification systems in selecting thyroid nodules for FNA. J Clin Endocrinol Metab. 2020; 105:dgz170.
Article35. Kim PH, Suh CH, Baek JH, Chung SR, Choi YJ, Lee JH. Unnecessary thyroid nodule biopsy rates under four ultrasound risk stratification systems: a systematic review and meta-analysis. Eur Radiol. 2021; 31:2877–85.
Article36. Ha EJ, Na DG, Baek JH. Korean thyroid imaging reporting and data system: current status, challenges, and future perspectives. Korean J Radiol. 2021; 22:1569–78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic performance of ultrasound risk stratification systems on thyroid nodules cytologically classified as indeterminate: a systematic review and meta-analysis
- Ultrasound Risk Stratification System of Thyroid Nodule
- Ultrasound Risk Stratification System of Thyroid Nodule
- Korean Thyroid Imaging Reporting and Data System: Current Status, Challenges, and Future Perspectives
- Thyroid Imaging Reporting and Data System (TIRADS)