Ann Surg Treat Res.
2023 Feb;104(2):90-100. 10.4174/astr.2023.104.2.90.
Expression levels of RUNX3 and FGFR2 in peripheral blood of severe acute pancreatitis and their clinical significance
- Affiliations
-
- 1Department of Critical Medicine, Dushu Lake Hospital Affiliated to Soochow University, Suzhou, China
- 2Department of Critical Medicine, Wuzhong People’s Hospital, Suzhou, China
- KMID: 2539223
- DOI: http://doi.org/10.4174/astr.2023.104.2.90
Abstract
- Purpose
Severe acute pancreatitis (SAP) is a life-threatening inflammatory syndrome of the pancreas. This study aimed to analyze the clinical significance of runt-associated transcription factor 3 (RUNX3) and fibroblast growth factor receptor 2 (FGFR2) expression alterations in SAP.
Methods
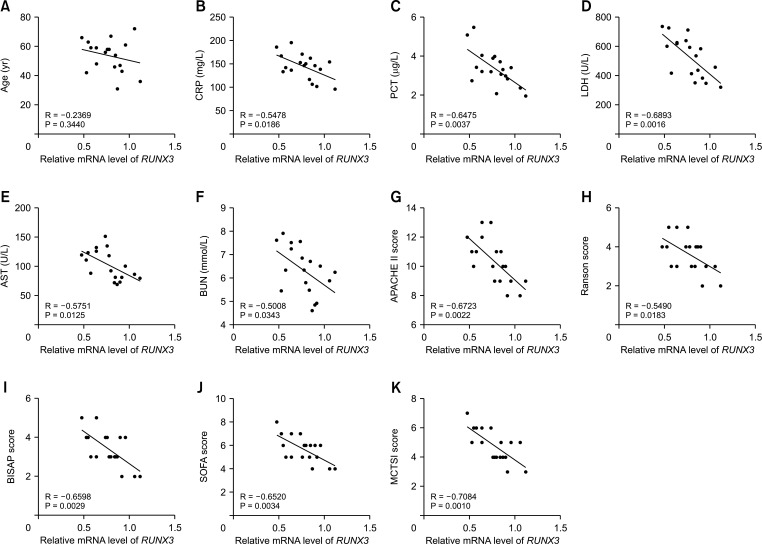
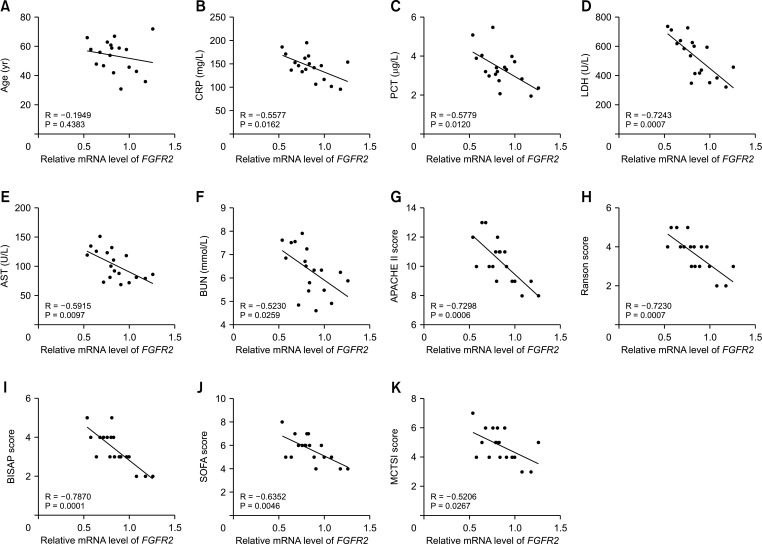
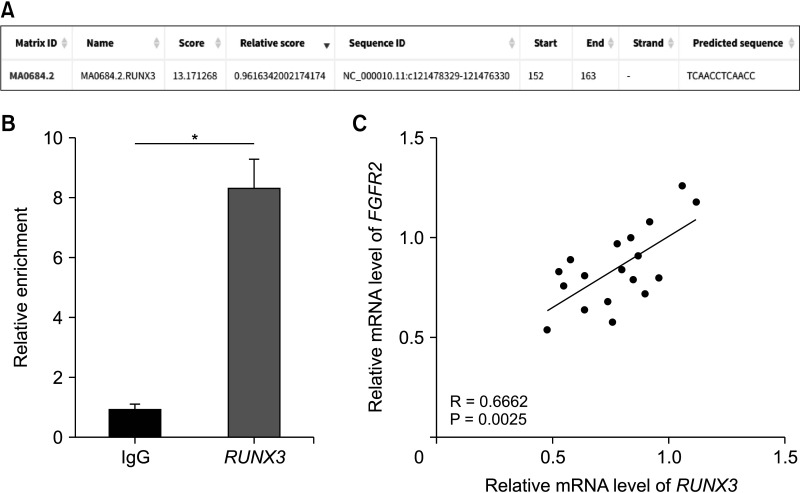
This study included 18 SAP patients in Wuzhong People’s Hospital from November 2019 to December 2021 and 18 healthy controls. RUNX3 and FGFR2 expression levels were determined by RT-quantitative PCR. Correlations between RUNX3/FGFR2 and sex, age, etiology, CRP, procalcitonin, AST, LDH, BUN, Acute Physiology and Chronic Health Evaluation II (APACHE II), Ranson score, Bedside Index for Severity in Acute Pancreatitis (BISAP) score, sequential organ failure assessment (SOFA), and modified computed tomography severity index (MCTSI) score were analyzed. Diagnostic values of RUNX3 and FGFR2 in SAP were analyzed using the receiver-operating characteristic curve. The binding of RUNX3 to FGFR2 was analyzed by chromatin immunoprecipitation.
Results
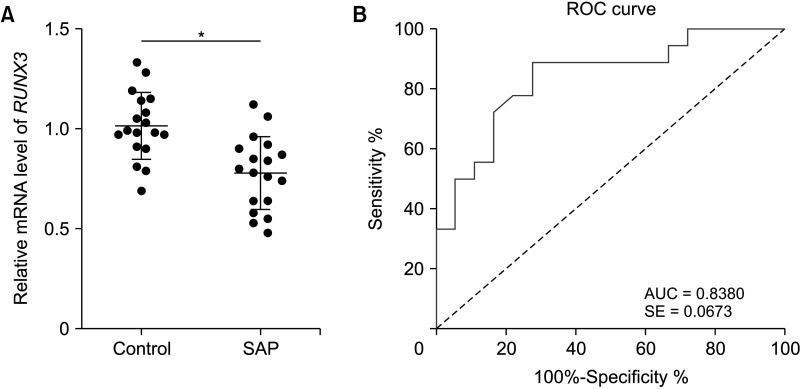
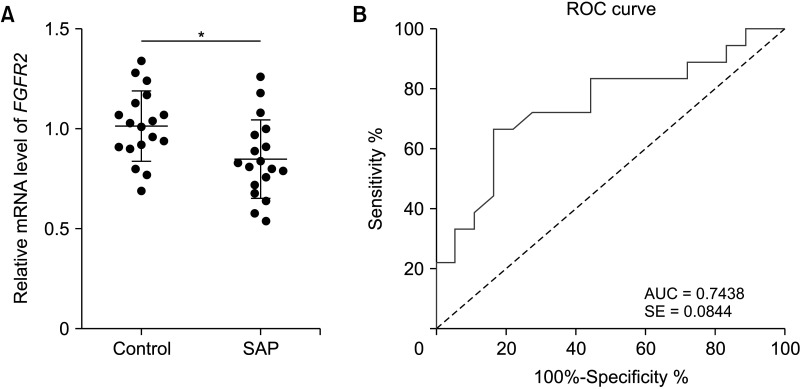
RUNX3 and FGFR2 were downregulated in peripheral blood of SAP patients. RUNX3 and FGFR2 were negatively correlated with CRP, procalcitonin, AST, LDH, BUN, APACHE II score, Ranson score, BISAP score, SOFA score, and MCTSI score. Sensitivity and specificity of RUNX3 level of <0.9650 for SAP diagnosis were 88.89% and 72.22%, respectively. Sensitivity and specificity of FGFR2 level of <0.8950 for SAP diagnosis were 66.67% and 83.33%, respectively. RUNX3 was enriched in the FGFR2 promoter and was positively correlated with FGFR2.
Conclusion
RUNX3 and FGFR2 were downregulated in peripheral blood of SAP patients and served as candidate biomarkers for SAP diagnosis. RUNX3 bound to the FGFR2 promoter to promote FGFR2 transcription.
Keyword
Figure
Reference
-
1. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020; 396:726–734. PMID: 32891214.2. Gliem N, Ammer-Herrmenau C, Ellenrieder V, Neesse A. Management of severe acute pancreatitis: an update. Digestion. 2021; 102:503–507. PMID: 32422634.3. Hines OJ, Pandol SJ. Management of severe acute pancreatitis. BMJ. 2019; 367:l6227. PMID: 31791953.4. Ercan G, İlbar Tartar R, Solmaz A, Gulcicek OB, Karagulle OO, Meric S, et al. Examination of protective and therapeutic effects of ruscogenin on cerulein-induced experimental acute pancreatitis in rats. Ann Surg Treat Res. 2019; 97:271–281. PMID: 31824881.5. Lotem J, Levanon D, Negreanu V, Bauer O, Hantisteanu S, Dicken J, et al. Runx3 in immunity, inflammation and cancer. Adv Exp Med Biol. 2017; 962:369–393. PMID: 28299669.6. Pang L, Yu P, Liu X, Fan Y, Shi Y, Zou S. Fine particulate matter induces airway inflammation by disturbing the balance between Th1/Th2 and regulation of GATA3 and Runx3 expression in BALB/c mice. Mol Med Rep. 2021; 23:378. PMID: 33760131.7. Dybska E, Adams AT, Duclaux-Loras R, Walkowiak J, Nowak JK. Waiting in the wings: RUNX3 reveals hidden depths of immune regulation with potential implications for inflammatory bowel disease. Scand J Immunol. 2021; 93:e13025. PMID: 33528856.8. Li S, Cui HZ, Xu CM, Sun ZW, Tang ZK, Chen HL. RUNX3 protects against acute lung injury by inhibiting the JAK2/STAT3 pathway in rats with severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2019; 23:5382–5391. PMID: 31298391.9. ZhuGe DL, Javaid HM, Sahar NE, Zhao YZ, Huh JY. Fibroblast growth factor 2 exacerbates inflammation in adipocytes through NLRP3 inflammasome activation. Arch Pharm Res. 2020; 43:1311–1324. PMID: 33245516.10. Wang Y, Shi T, Wang X, Hu J, Yu L, Liu Q, et al. FGFR2 alteration as a potential therapeutic target in poorly cohesive gastric carcinoma. J Transl Med. 2021; 19:401. PMID: 34551773.11. Nakada S, Tsuneyama K, Kato I, Tabuchi Y, Takasaki I, Furusawa Y, et al. Identification of candidate genes involved in endogenous protection mechanisms against acute pancreatitis in mice. Biochem Biophys Res Commun. 2010; 391:1342–1347. PMID: 20026013.12. Yang Q, Wang Y, Li M, Wang Z, Zhang J, Dai W, et al. HMGA1 promotes gastric cancer growth and metastasis by transactivating SUZ12 and CCDC43 expression. Aging (Albany NY). 2021; 13:16043–16061. PMID: 34167089.13. Huang K, Yang C, Zheng J, Liu X, Liu J, Che D, et al. Effect of circular RNA, mmu_circ_0000296, on neuronal apoptosis in chronic cerebral ischaemia via the miR-194-5p/Runx3/Sirt1 axis. Cell Death Discov. 2021; 7:124. PMID: 34052838.14. Zhang YP, Liu C, Ye L, Yu N, Ye YN, Sun WR, et al. Early prediction of persistent organ failure by serum angiopoietin-2 in patients with acute pancreatitis. Dig Dis Sci. 2016; 61:3584–3591. PMID: 27686934.15. Wilson C, Heath DI, Imrie CW. Prediction of outcome in acute pancreatitis: a comparative study of APACHE II, clinical assessment and multiple factor scoring systems. Br J Surg. 1990; 77:1260–1264. PMID: 2253005.16. Yin X, Zhong X, Li J, Le M, Shan S, Zhu C. The value of RANSON score combined with BMI in predicting the mortality in severe acute pancreatitis: a retrospective study. Int J Gen Med. 2022; 15:5015–5025. PMID: 35607358.17. Bezmarević M, Kostić Z, Jovanović M, Micković S, Mirković D, Soldatović I, et al. Procalcitonin and BISAP score versus C-reactive protein and APACHE II score in early assessment of severity and outcome of acute pancreatitis. Vojnosanit Pregl. 2012; 69:425–431. PMID: 22764546.18. Hoß KF, Attenberger UI. [Classification of pancreatitis]. Radiologe. 2021; 61:524–531. German. PMID: 33988737.19. Mikó A, Vigh É, Mátrai P, Soós A, Garami A, Balaskó M, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. Front Physiol. 2019; 10:1002. PMID: 31507427.20. Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020; 48(D1):D87–D92. PMID: 31701148.21. Habashy DM, Eissa DS, Aboelez MM. Cryptochrome-1 gene expression is a reliable prognostic indicator in egyptian patients with chronic lymphocytic leukemia: a prospective cohort study. Turk J Haematol. 2018; 35:168–174. PMID: 28884705.22. Li X, Cao Y, Liu Z, Chen H, Mao H. The relationship between liver injury and serum levels of C-reactive protein and procalcitonin in patients with acute pancreatitis. J Coll Physicians Surg Pak. 2019; 29:287–289. PMID: 30823962.23. Liang Y, Zhao X, Meng F. Procalcitonin, C-reactive protein, and neutrophil ratio contribute to the diagnosis and prognosis of severe acute pancreatitis. Iran J Public Health. 2019; 48:2177–2186. PMID: 31993385.24. Basit H, Ruan GJ, Mukherjee S. Ranson criteria. StatPearls. Treasure Island (FL): StatPearls Publishing;2022.25. Wang Y, Yang X, Jiang A, Wang W, Li J, Wen J. Methylation-dependent transcriptional repression of RUNX3 by KCNQ1OT1 regulates mouse cardiac microvascular endothelial cell viability and inflammatory response following myocardial infarction. FASEB J. 2019; 33:13145–13160. PMID: 31625414.26. Whittle MC, Hingorani SR. Runx3 and cell fate decisions in pancreas cancer. Adv Exp Med Biol. 2017; 962:333–352. PMID: 28299667.27. Sugai M, Aoki K, Osato M, Nambu Y, Ito K, Taketo MM, et al. Runx3 is required for full activation of regulatory T cells to prevent colitis-associated tumor formation. J Immunol. 2011; 186:6515–6520. PMID: 21515792.28. Wiedlocha A, Haugsten EM, Zakrzewska M. Roles of the FGF-FGFR signaling system in cancer development and inflammation. Cells. 2021; 10:2231. PMID: 34571880.29. Chen J, Wang Z, Zheng Z, Chen Y, Khor S, Shi K, et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death Dis. 2017; 8:e3090. PMID: 28981091.30. Jang SY, Choi SH, Kikkawa D, Lee EJ, Yoon JS. Association of fibroblast growth factor 10 with the fibrotic and inflammatory pathogenesis of Graves’ orbitopathy. PLoS One. 2021; 16:e0255344. PMID: 34383782.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of RUNX3 in Human Gastric Cancer
- CD64 Expression Is Increased in Patients with Severe Acute Pancreatitis: Clinical Significance
- Assessment of Severity and Fluid Administration in Acute Pancreatitis
- DNA Methylation of RUNX3 in Papillary Thyroid Cancer
- Medical Management of Acute Pancreatitis and Complications