Intest Res.
2023 Jan;21(1):110-125. 10.5217/ir.2021.00143.
Efficacy and safety of filgotinib as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a post-hoc analysis of the phase 2b/3 SELECTION trial
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Hokkaido Prefectural Welfare Federation of Agricultural Cooperatives, Sapporo-Kosei General Hospital, Sapporo, Japan
- 3Kyorin University School of Medicine, Tokyo, Japan
- 4Fukuoka University, Fukuoka, Japan
- 5Hyogo College of Medicine, Nishinomiya, Japan
- 6Toho University Sakura Medical Center, Sakura, Japan
- 7The Jikei University School of Medicine, Tokyo, Japan
- 8Alimentiv Inc., London, ON, Canada
- 9Western University, London, ON, Canada
- 10Galapagos NV, Mechelen, Belgium
- 11Galapagos NV, Leiden, Netherlands
- 12Gilead Sciences, Inc., Foster City, CA, USA
- 13Gilead Sciences K.K., Tokyo, Japan
- 14Tokyo Medical and Dental University, Tokyo, Japan
- KMID: 2539010
- DOI: http://doi.org/10.5217/ir.2021.00143
Abstract
- Background/Aims
The safety and efficacy of filgotinib, a once-daily oral Janus kinase 1 preferential inhibitor, were evaluated in Japanese patients with ulcerative colitis (UC) in the phase 2b/3 SELECTION trial.
Methods
SELECTION (NCT02914522) was a randomized, placebo-controlled trial comprising 2 induction studies and a maintenance study. Adults with moderately to severely active UC were randomized in induction study A (biologic-naïve) or B (biologic-experienced) to receive filgotinib 200 mg, 100 mg, or placebo once daily for 11 weeks. Patients in clinical remission or Mayo Clinic score response at week 10 entered the 47-week maintenance study. Efficacy and safety outcomes were assessed in Japanese patients enrolled in Japan.
Results
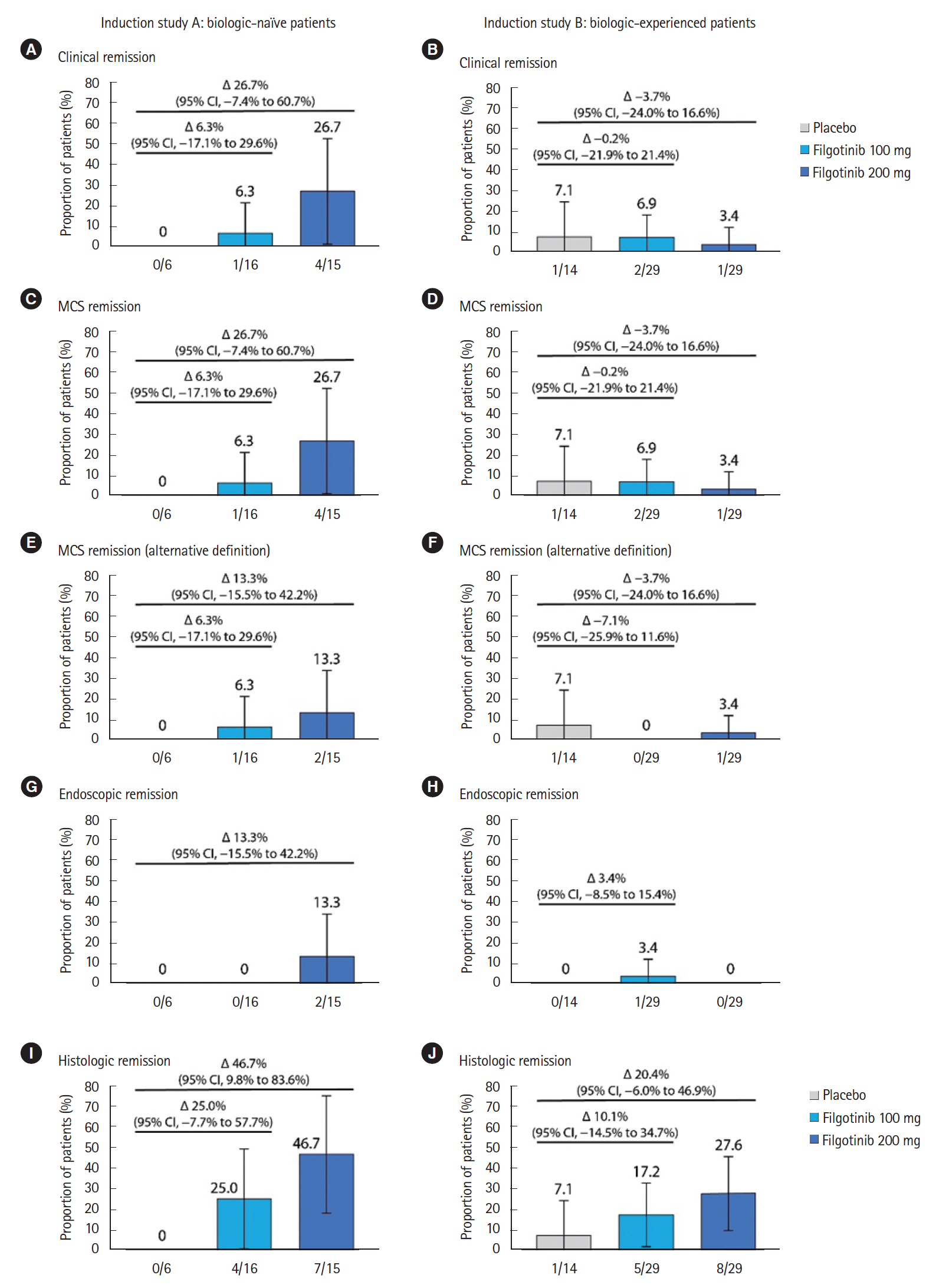
Overall, 37 and 72 Japanese patients were enrolled in Japan in induction studies A and B, respectively, and 54 entered the maintenance study. Numerically higher proportions of filgotinib 200 mg-treated than placebo-treated patients achieved clinical remission in induction study A (4/15 [26.7%] vs. 0/6 [0%]) and the maintenance study (5/20 [25.0%] vs. 0/9 [0%]), but not induction study B (1/29 [3.4%] vs. 1/14 [7.1%]). Both doses were well tolerated, and no new safety signals were noted. Herpes zoster was reported in 1 filgotinib 200 mg-treated patient in each of induction study A (2.3%, 1/44) and the maintenance study (5.0%, 1/20).
Conclusions
These data, alongside those of the overall SELECTION population, suggest the potential of filgotinib 200 mg as a viable treatment option for Japanese patients with UC. Owing to small patient numbers, data should be interpreted cautiously.
Figure
Cited by 1 articles
-
The role and prospect of tofacitinib in patients with ulcerative colitis
Jun Lee
Intest Res. 2023;21(1):168-169. doi: 10.5217/ir.2022.00098.
Reference
-
1. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020; 6:74.
Article2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article3. Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004; 53:1190–1197.
Article4. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article5. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.
Article6. Kanatani Y, Tomita N, Sato Y, Eto A, Omoe H, Mizushima H. National registry of designated intractable diseases in Japan: present status and future prospects. Neurol Med Chir (Tokyo). 2017; 57:1–7.
Article7. Murakami Y, Nishiwaki Y, Oba MS, et al. Correction to: Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2015: an analysis of a nationwide survey. J Gastroenterol. 2020; 55:131.
Article8. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article9. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015; 110:1324–1338.10. Fukunaga K, Matsumoto T. Current status and future perspectives of leukocytapheresis for inflammatory bowel disease. J Gastroenterol Hepatol. 2012; 27:997–1003.
Article11. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article12. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article13. Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review with network meta-analysis: first- and second-line pharmacotherapy for moderate-severe ulcerative colitis. Aliment Pharmacol Ther. 2018; 47:162–175.
Article14. Ashton JJ, Green Z, Kolimarala V, Beattie RM. Inflammatory bowel disease: long-term therapeutic challenges. Expert Rev Gastroenterol Hepatol. 2019; 13:1049–1063.
Article15. Salas A, Hernandez-Rocha C, Duijvestein M, et al. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020; 17:323–337.
Article16. Dowty ME, Lin TH, Jesson MI, et al. Janus kinase inhibitors for the treatment of rheumatoid arthritis demonstrate similar profiles of in vitro cytokine receptor inhibition. Pharmacol Res Perspect. 2019; 7:e00537.
Article17. Choy EH. Clinical significance of Janus kinase inhibitor selectivity. Rheumatology (Oxford). 2019; 58:953–962.
Article18. Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019; 68:1893–1899.
Article19. Di Paolo J, Downie B, Meng A, Mollova N, Yu Y, Han P. Evaluation of potential mechanisms underlying the safety observations of filgotinib in clinical studies in RA. Arthritis Rheumatol. 2019; 71(Suppl 10):59.20. European Medicines Agency. Filgotinib European public assessment report [Internet]. c2021 [cited 2021 Aug 05]. https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca.21. Pharmaceuticals and Medical Devices Agency. Approval of filgotinib in Japan [Internet]. c2021 [cited 2021 Aug 05]. https://www.pmda.go.jp/files/000239965.pdf.22. Namour F, Vayssière B, Galien R, et al. AB0494 Filgotinib (GLPG0634), a selective JAK1 inhibitor, shows similar PK and PD profiles in Japanese and Caucasian healthy volunteers. Ann Rheum Dis. 2015; 74(Suppl 2):1063–1064.23. Feagan BG, Danese S, Loftus EV Jr, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021; 397:2372–2384.
Article24. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article25. Lemmens B, Arijs I, Van Assche G, et al. Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm Bowel Dis. 2013; 19:1194–1201.
Article26. Electronic Medicines Compendium. Filgotinib Summary of Product Characteristics [Internet]. c2021 [cited 2021 Nov 15]. https://www.medicines.org.uk/emc/product/2500/smpc.27. Winthrop KL, Nash P, Yamaoka K, et al. Incidence and risk factors for herpes zoster in patients with rheumatoid arthritis receiving upadacitinib: a pooled analysis of six phase III clinical trials. Ann Rheum Dis. 2022; 81:206–213.
Article28. U.S. Food and Drug Administration. Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions [Internet]. c2021 [cited 2021 Nov 16]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.29. Gilead Sciences, Inc. Jyseleca Japan interview form [Internet]. c2021 [cited 2021 Nov 17]. https://www.jyseleca.jp/-/media/Files/Filgotinib/product/basic/jys_if.pdf.30. D’Amico F, Magro F, Peyrin-Biroulet L, Danese S. Positioning filgotinib in the treatment algorithm of moderate to severe ulcerative colitis. J Crohns Colitis. 2022; 16:835–844.
Article31. Richez C, Truchetet ME. Evaluating filgotinib for the treatment of rheumatoid arthritis. Expert Opin Pharmacother. 2021; 22:2435–2444.
Article32. Namour F, Desrivot J, Van der Aa A, Harrison P, Tasset C, van’t Klooster G. Clinical confirmation that the selective JAK1 inhibitor filgotinib (GLPG0634) has a low liability for drug-drug interactions. Drug Metab Lett. 2016; 10:38–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Filgotinib induction-study baseline characteristics of patients with ulcerative colitis who achieve sustained corticosteroid-free remission: post hoc analysis of the phase 2b/3 SELECTION study
- Efficacy and safety of mirikizumab as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a subgroup analysis of the global phase 3 LUCENT-1 and LUCENT-2 studies
- Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn's disease: a subpopulation analysis of phase 3 induction and maintenance studies
- Efficacy and Safety of Adalimumab in Moderately to Severely Active Cases of Ulcerative Colitis: A Meta-Analysis of Published Placebo-Controlled Trials
- Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis