Intest Res.
2023 Jan;21(1):88-99. 10.5217/ir.2021.00154.
Incidence rates for hospitalized infections, herpes zoster, and malignancies in patients with ulcerative colitis in Japan: an administrative health claims database analysis
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan
- 2Pfizer Japan Inc., Tokyo, Japan
- KMID: 2539008
- DOI: http://doi.org/10.5217/ir.2021.00154
Abstract
- Background/Aims
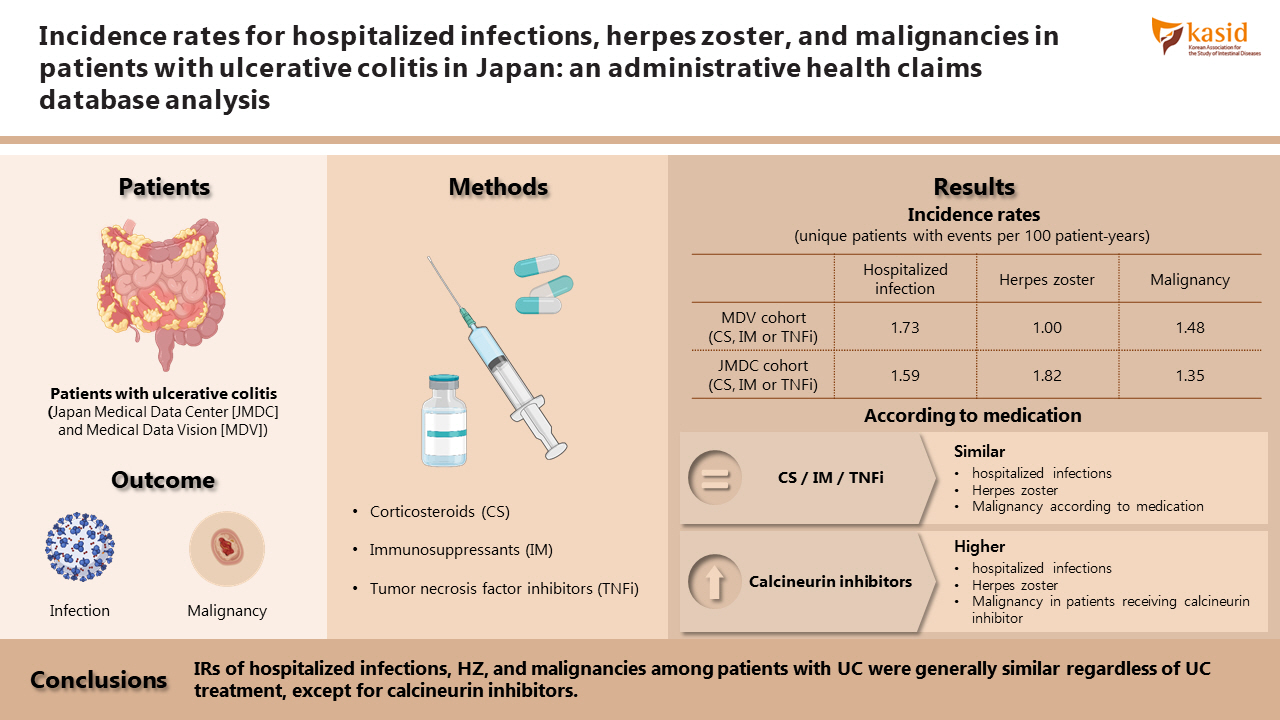
Patients with ulcerative colitis (UC) are at an increased risk of certain infections and malignancies compared with the general population. Incidence rates (IRs) of hospitalized infections, herpes zoster (HZ), and malignancies in patients with UC, stratified by treatment, in Japan were estimated.
Methods
This retrospective study identified patients with UC treated with corticosteroids, immunosuppressants, or tumor necrosis factor inhibitors (TNFi) from 2 administrative databases (Japan Medical Data Center [JMDC] and Medical Data Vision [MDV]). IRs (unique patients with events per 100 patient‐years) were estimated for hospitalized infections, HZ, and malignancies, between June 2010 and May 2018.
Results
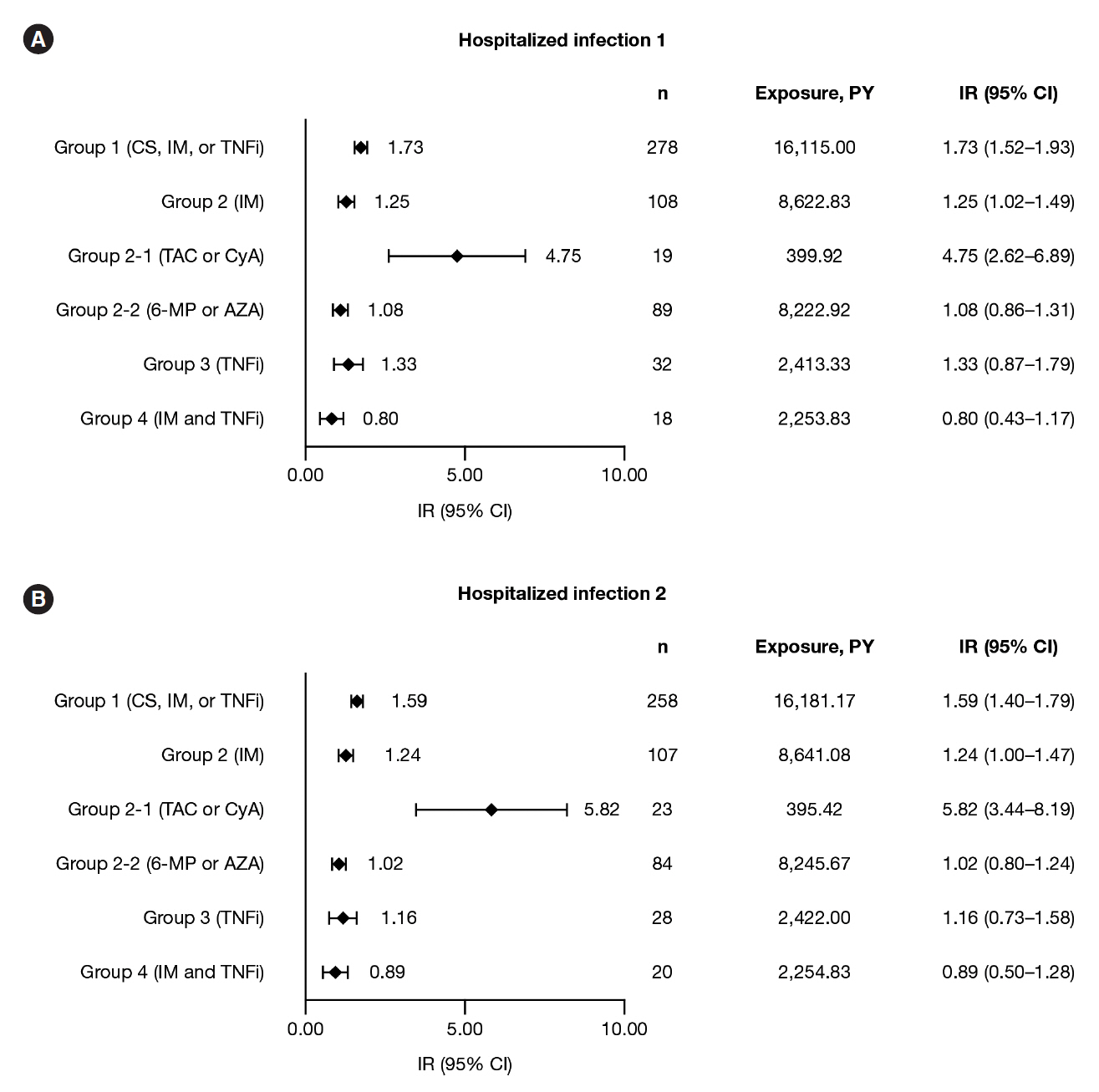
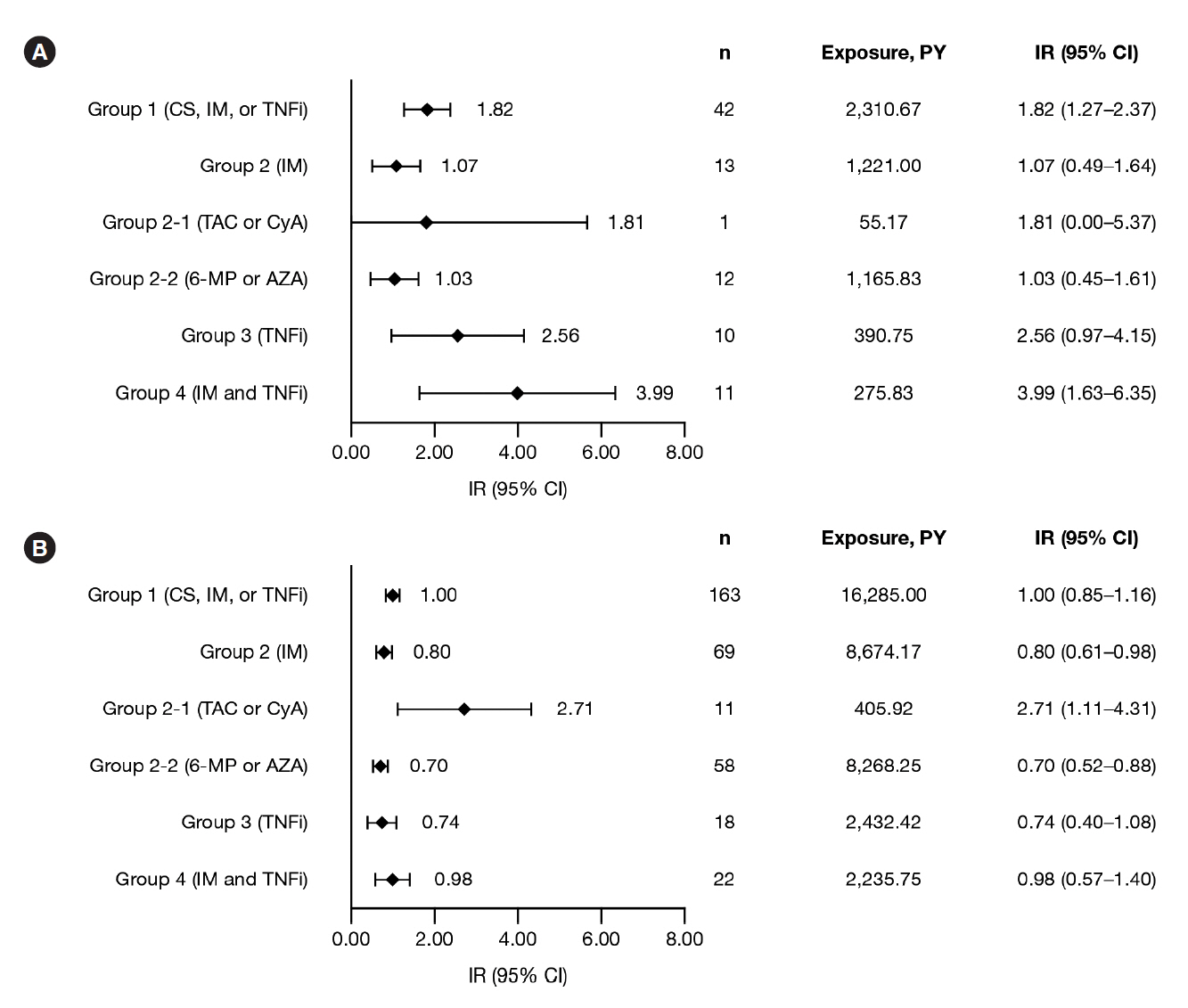
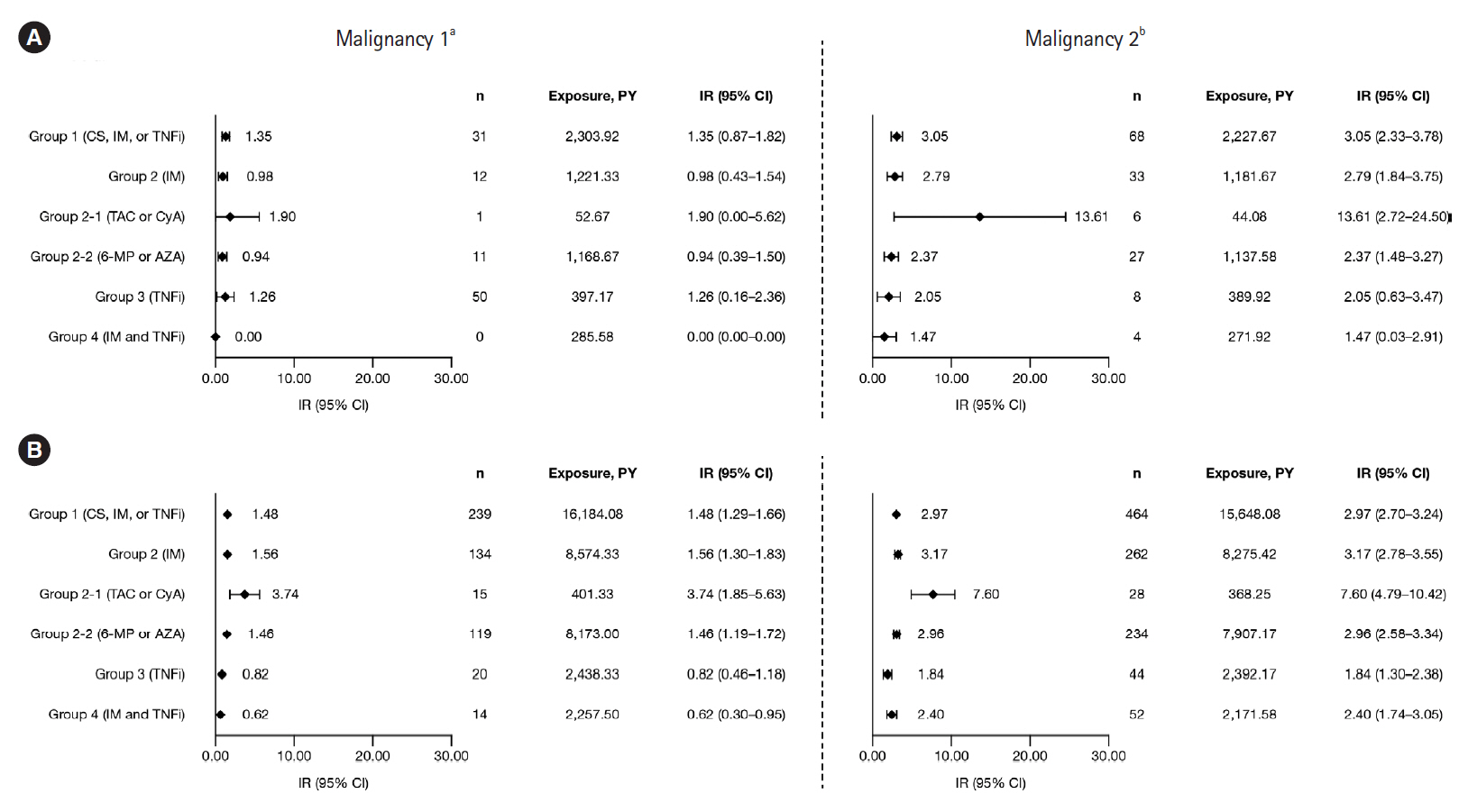
Among 6,033 MDV patients with UC receiving corticosteroids, immunosuppressants, or TNFi, IRs (95% confidence intervals) were: hospitalized infections, 1.73 (1.52–1.93); HZ, 1.00 (0.85–1.16), and malignancies, 1.48 (1.29–1.66). Among 958 JMDC patients with UC receiving corticosteroids, immunosuppressants, or TNFi, IRs (95% confidence intervals) were: HZ, 1.82 (1.27–2.37) and malignancies, 1.35 (0.87–1.82). In both cohorts, IRs of malignancies were generally similar among patients receiving immunosuppressants, TNFi, or combination therapy (immunosuppressants and TNFi); this was also true for IRs of hospitalized infections and HZ in the MDV cohort. IRs of hospitalized infections, HZ, and malignancies were higher in patients receiving calcineurin inhibitors compared with immunosuppressants or TNFi, in both cohorts.
Conclusions
IRs of hospitalized infections, HZ, and malignancies among patients with UC were generally similar regardless of UC treatment, except for calcineurin inhibitors.
Keyword
Figure
Reference
-
1. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012; 380:1606–1619.
Article2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article3. Morita N, Toki S, Hirohashi T, et al. Incidence and prevalence of inflammatory bowel disease in Japan: nationwide epidemiological survey during the year 1991. J Gastroenterol. 1995; 30 Suppl 8:1–4.4. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.
Article5. Kappelman MD, Farkas DK, Long MD, et al. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014; 12:265–273.6. Kantsø B, Simonsen J, Hoffmann S, Valentiner-Branth P, Petersen AM, Jess T. Inflammatory bowel disease patients are at increased risk of invasive pneumococcal disease: a nationwide Danish cohort study 1977-2013. Am J Gastroenterol. 2015; 110:1582–1587.
Article7. Long MD, Kappelman MD, Pipkin CA. Nonmelanoma skin cancer in inflammatory bowel disease: a review. Inflamm Bowel Dis. 2011; 17:1423–1427.8. Beaugerie L, Rahier JF, Kirchgesner J. Predicting, preventing, and managing treatment-related complications in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020; 18:1324–1335.
Article9. Imafuku S, Dormal G, Goto Y, Jégou C, Rosillon D, Matsuki T. Risk of herpes zoster in the Japanese population with immunocompromising and chronic disease conditions: results from a claims database cohort study, from 2005 to 2014. J Dermatol. 2020; 47:236–244.
Article10. Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016; 68:2328–2337.
Article11. Gupta G, Lautenbach E, Lewis JD. Incidence and risk factors for herpes zoster among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006; 4:1483–1490.
Article12. Khan N, Patel D, Trivedi C, et al. Overall and comparative risk of herpes zoster with pharmacotherapy for inflammatory bowel diseases: a nationwide cohort study. Clin Gastroenterol Hepatol. 2018; 16:1919–1927.
Article13. Craviotto V, Furfaro F, Loy L, et al. Viral infections in inflammatory bowel disease: tips and tricks for correct management. World J Gastroenterol. 2021; 27:4276–4297.
Article14. Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of herpes zoster among 108 604 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 37:420–429.
Article15. Chang K, Lee HS, Kim YJ, et al. Increased risk of herpes zoster infection in patients with inflammatory bowel diseases in Korea. Clin Gastroenterol Hepatol. 2018; 16:1928–1936.
Article16. Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015; 9:945–965.
Article17. Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013; 177:1296–1305.
Article18. Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011; 106:2146–2153.
Article19. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017; 318:1679–1686.
Article20. Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2014; 43:525–541.
Article21. Park SC, Jeen YT. Genetic studies of inflammatory bowel disease-focusing on Asian patients. Cells. 2019; 8:404.
Article22. Nakase H, Uchino M, Shinzaki S, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021; 56:489–526.
Article23. Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017; 2:269–276.
Article24. Nagai K, Tanaka T, Kodaira N, Kimura S, Takahashi Y, Nakayama T. Data resource profile: JMDC claims databases sourced from Medical Institutions. J Gen Fam Med. 2020; 21:211–218.
Article25. Ogino H, Morikubo H, Fukaura K, et al. Validation of a claimsbased algorithm to identify cases of ulcerative colitis in Japan. J Gastroenterol Hepatol. 2022; 37:499–506.
Article26. Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare. Ethical guidelines for medical and health research involving human subjects [Internet]. c2018 [cited 2021 Oct 01]. https://www.lifescience.mext.go.jp/files/pdf/n2181_01.pdf.27. Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018; 155:337–346.
Article28. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol. 2012; 107:1409–1422.
Article29. Nyboe Andersen N, Pasternak B, Friis-Møller N, Andersson M, Jess T. Association between tumour necrosis factor-α inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ. 2015; 350–h2809.
Article30. Kirchgesner J, Desai RJ, Beaugerie L, Schneeweiss S, Kim SC. Risk of serious infections with vedolizumab versus tumor necrosis factor antagonists in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022; 20:314–324.
Article31. Curtis JR, Regueiro M, Yun H, et al. Tofacitinib treatment safety in moderate to severe ulcerative colitis: comparison of observational population cohort data from the IBM MarketScan® administrative claims database with tofacitinib trial data. Inflamm Bowel Dis. 2021; 27:1394–1408.
Article32. Imafuku S, Matsuki T, Mizukami A, et al. Burden of herpes zoster in the Japanese population with immunocompromised/chronic disease conditions: results from a cohort study claims database from 2005-2014. Dermatol Ther (Heidelb). 2019; 9:117–133.
Article33. de Luise C, Sugiyama N, Morishima T, et al. Validity of claimsbased algorithms for selected cancers in Japan: results from the VALIDATE-J study. Pharmacoepidemiol Drug Saf. 2021; 30:1153–1161.
Article34. Kobayashi T, Uda A, Udagawa E, Hibi T. Lack of increased risk of lymphoma by thiopurines or biologics in Japanese patients with inflammatory bowel disease: a large-scale administrative database analysis. J Crohns Colitis. 2020; 14:617–623.
Article35. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparative Risk of Serious Adverse Events With Tofacitinib and TNF Inhibitors in Patients With Ulcerative Colitis: The Korean Experience as Revealed by a National Database
- A Study of Cellular and Humoral Immunity in Patients with Herpes Zoster
- A Case of Human Immunodeficiency Virus Infection in Recurrent Herpes Zoster
- Herpes Zoster in the Patients with Malignant Tumor
- Association between oral corticosteroid starting dose and the incidence of pneumonia in Japanese patients with ulcerative colitis: a nation-wide claims database study