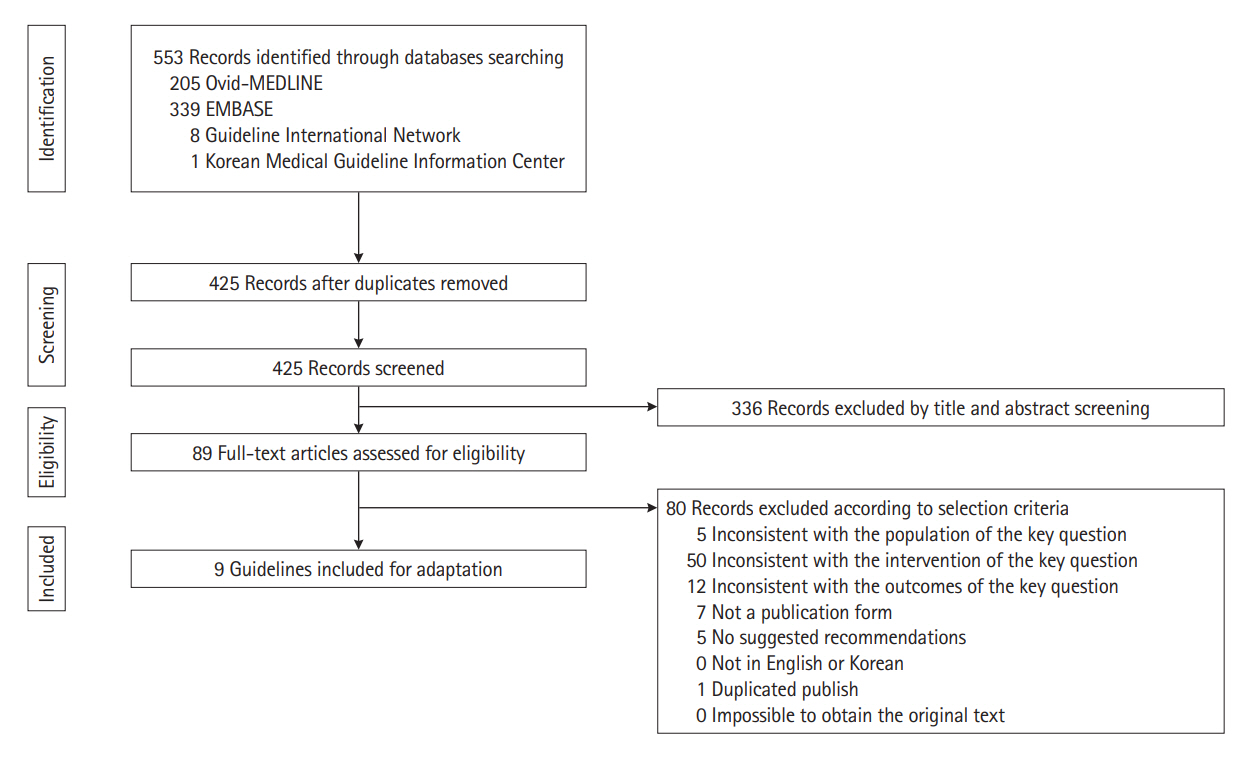
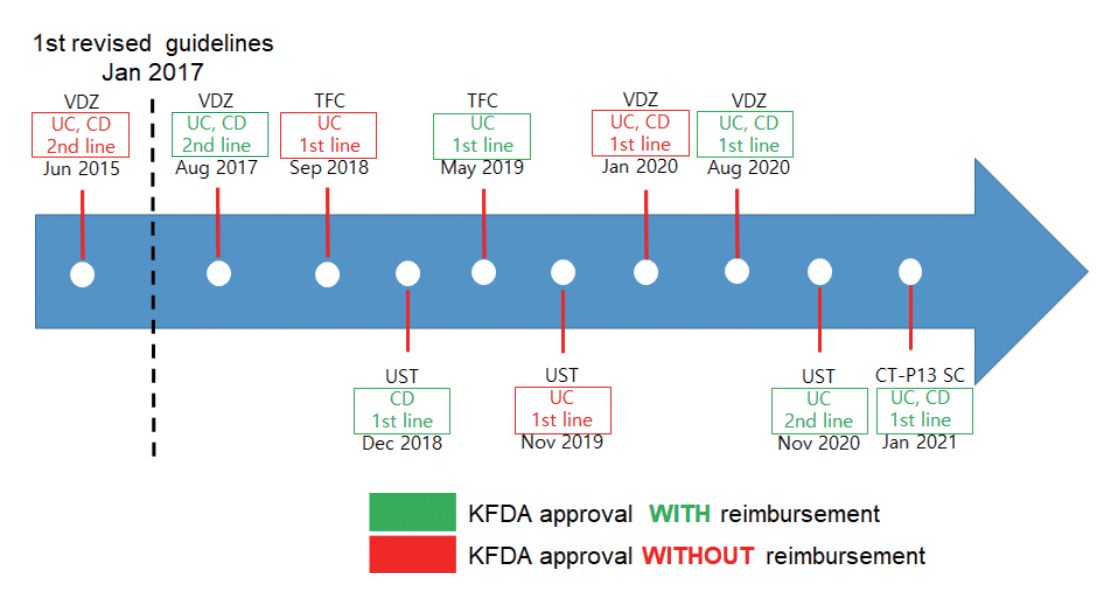
Intest Res.
2023 Jan;21(1):61-87. 10.5217/ir.2022.00007.
Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis
- Affiliations
-
- 1Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 2Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 5Department of Gastroenterology and Inflammatory Bowel Disease Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- 7Department of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 9Department of Gastroenterology, College of Medicine, Kyung Hee University, Seoul, Korea
- 10Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 11Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 13National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- KMID: 2539007
- DOI: http://doi.org/10.5217/ir.2022.00007
Abstract
- Ulcerative colitis (UC), a relapsing-remitting chronic inflammatory bowel disease (IBD), has a variable natural course but potentially severe disease course. Since the development of anti-tumor necrosis factor (TNF) agents has changed the natural disease course of moderate-to-severe UC, therapeutic options for patients who failed conventional treatments are expanding rapidly. IBD clinical trials have demonstrated the potential efficacy and safety of novel biologics such as anti-integrin α4β7 and anti-interleukin-12/23 monoclonal antibodies and small molecules such as a Janus kinase inhibitor. Anti-TNF biosimilars also have been approved and are widely used in IBD patients. Wise drug choices should be made considering evidence-based efficacy and safety. However, the best position of these drugs remains several questions, with limited data from direct comparative trials. In addition, there are still concerns to be elucidated on the effect of therapeutic drug monitoring and combination therapy with immunomodulators. The appropriate treatment regimens in acute severe UC and the risk of perioperative use of biologics are unclear. As novel biologics and small molecules have been approved in Korea, we present the Korean guidelines for medical management of adult outpatients with moderate-to-severe UC and adult hospitalized patients with acute severe UC, focusing on biologics and small molecules.
Figure
Cited by 4 articles
-
The role and prospect of tofacitinib in patients with ulcerative colitis
Jun Lee
Intest Res. 2023;21(1):168-169. doi: 10.5217/ir.2022.00098.Safety of Biologic Therapy in Older Adults with Inflammatory Bowel Diseases
Tae-Geun Gweon
Korean J Gastroenterol. 2023;81(5):230-232. doi: 10.4166/kjg.2023.047.How have treatment patterns for patients with inflammatory bowel disease changed in Asian countries?
Jihye Park
Intest Res. 2023;21(3):275-276. doi: 10.5217/ir.2023.00061.Treatment of primary sclerosing cholangitis combined with inflammatory bowel disease
You Sun Kim, Edward H. Hurley, Yoojeong Park, Sungjin Ko
Intest Res. 2023;21(4):420-432. doi: 10.5217/ir.2023.00039.
Reference
-
1. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the SongpaKangdong district of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019; 13:1410–1417.
Article2. Ekbom A, Helmick C, Zack M, Adami HO. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology. 1991; 100:350–358.
Article3. Moum B, Vatn MH, Ekbom A, et al. Incidence of ulcerative colitis and indeterminate colitis in four counties of southeastern Norway, 1990-93: a prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. Scand J Gastroenterol. 1996; 31:362–366.
Article4. Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol. 2009; 44:431–440.
Article5. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018; 16:343–356.
Article6. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013; 145:996–1006.
Article7. Cha JM, Park SH, Rhee KH, et al. Long-term prognosis of ulcerative colitis and its temporal changes between 1986 and 2015 in a population-based cohort in the Songpa-Kangdong district of Seoul, Korea. Gut. 2020; 69:1432–1440.
Article8. Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009; 137:1250–1260.
Article9. Feagan BG, Sandborn WJ, Lazar A, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014; 146:110–118.
Article10. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010; 4:355–366.
Article11. D’Haens GR, Panaccione R, Higgins PD, et al. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011; 106:199–212.
Article12. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011; 33:987–995.
Article13. Andersen NN, Jess T. Risk of infections associated with biological treatment in inflammatory bowel disease. World J Gastroenterol. 2014; 20:16014–16019.
Article14. Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009; 7:874–881.
Article15. Choi CH, Kim YH, Kim YS, et al. Guidelines for the management of ulcerative colitis. Intest Res. 2012; 10:1–25.
Article16. Choi CH, Moon W, Kim YS, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017; 15:7–37.
Article17. Na SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver. 2019; 13:604–616.
Article18. Korean Medical Guideline Information Center. K-AGREE II (Korean Appraisal of Guidelines for Research and Evaluation II). c2008 [cited 2021 Jan 7]. https://www.guideline.or.kr/evaluation/file/K-AGREE%20II%20_01.pdf.19. Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1450–1461.
Article20. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019; 114:384–413.
Article21. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019; 68(Suppl 3):s1–s106.
Article22. National Institute for Health and Care Excellence (NICE). Ulcerative colitis: management [Internet]. c2019 [cited 2020 Dec 23]. www.nice.org.uk/guidance/ng130.23. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article24. Ooi CJ, Hilmi I, Banerjee R, et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn’s disease in Asia. J Gastroenterol Hepatol. 2019; 34:1296–1315.
Article25. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook [Internet]. c2013 [cited 2021 Jan 7]. https://gdt.gradepro.org/app/handbook/handbook.html.26. Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.27. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article28. Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015; 149:238–245.
Article29. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article30. Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:590–599.
Article31. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.
Article32. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016; 43:482–513.
Article33. Bossa F, Fiorella S, Caruso N, et al. Continuous infusion versus bolus administration of steroids in severe attacks of ulcerative colitis: a randomized, double-blind trial. Am J Gastroenterol. 2007; 102:601–608.
Article34. Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009; 104:760–767.
Article35. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011; 106:674–684.
Article36. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012; 91:635–646.
Article37. Brandse JF, Mathôt RA, van der Kleij D, et al. Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016; 14:251–258.
Article38. Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021; 385:1280–1291.
Article39. Click B, Regueiro M. A practical guide to the safety and monitoring of new IBD therapies. Inflamm Bowel Dis. 2019; 25:831–842.
Article40. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res. 2018; 16:17–25.
Article41. Park DI, Hisamatsu T, Chen M, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.
Article42. Deepak P, Stobaugh DJ, Ehrenpreis ED. Infectious complications of TNF-α inhibitor monotherapy versus combination therapy with immunomodulators in inflammatory bowel disease: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2013; 22:269–276.43. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017; 318:1679–1686.
Article44. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article45. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017; 66:839–851.
Article46. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article47. Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther. 2018; 48:65–77.
Article48. Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Eur Acad Dermatol Venereol. 2016; 30:1148–1158.
Article49. Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015; 14:706–714.50. Curtis JR, Schulze-Koops H, Takiya L, et al. Efficacy and safety of tofacitinib in older and younger patients with rheumatoid arthritis. Clin Exp Rheumatol. 2017; 35:390–400.51. Xie W, Huang Y, Xiao S, Sun X, Fan Y, Zhang Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis. 2019; 78:1048–1054.
Article52. Desai RJ, Pawar A, Khosrow-Khavar F, Weinblatt ME, Kim SC. Risk of venous thromboembolism associated with tofacitinib in patients with rheumatoid arthritis: a populationbased cohort study. Rheumatology (Oxford). 2021; 61:121–130.
Article53. U.S. Food and Drug Administration. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate [Internet]. c2019 [cited 2021 Sep 20]. https://www.fda.gov/drugs/drug-safety-and-availability/safety-trial-finds-risk-blood-clots-lungs-and-death-higher-dose-tofacitinib-xeljanz-xeljanz-xr.54. European Medicines Agency. Increased risk of blood clots in lungs and death with higher dose of Xeljanz (tofacitinib) for rheumatoid arthritis [Internet]. c2019 [cited 2021 Sep 20]. https://www.ema.europa.eu/en/news/increased-risk-blood-clots-lungs-death-higher-dose-xeljanz-tofacitinib-rheumatoid-arthritis.55. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022; 386:316–326.
Article56. U.S. Food and Drug Administration. Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions [Internet]. c2021 [cited 2021 Oct 13]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.57. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article58. Jiang XL, Cui HF, Gao J, Fan H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015; 49:582–588.
Article59. Xian-Janssen Pharmaceutical Ltd. A study to evaluate the effectiveness and safety of infliximab in Chinese patients with active ulcerative colitis [Internet]. c2015 [cited 2021 Feb 8]. https://clinicaltrials.gov/ct2/show/NCT01551290.60. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011; 60:780–787.
Article61. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012; 142:257–265.
Article62. Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2014; 49:283–294.
Article63. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95.
Article64. Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019; 14:e0212989.
Article65. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; 376:1723–1736.
Article66. Peyrin-Biroulet L, Van Assche G, Gómez-Ulloa D, et al. Systematic review of tumor necrosis factor antagonists in extraintestinal manifestations in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2017; 15:25–36.
Article67. Guillo L, D’Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for extra-intestinal manifestations of inflammatory bowel disease: a systematic literature review. J Crohns Colitis. 2021; 15:1236–1243.
Article68. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019; 381:1215–1226.
Article69. Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020; 18:2179–2191.
Article70. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2019; 25:1896–1905.
Article71. Singh S, Allegretti JR, Siddique SM, Terdiman JP. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020; 158:1465–1496.
Article72. Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146:392–400.
Article73. Mantzaris GJ, Sfakianakis M, Archavlis E, et al. A prospective randomized observer-blind 2-year trial of azathioprine monotherapy versus azathioprine and olsalazine for the maintenance of remission of steroid-dependent ulcerative colitis. Am J Gastroenterol. 2004; 99:1122–1128.
Article74. Singh S, Proudfoot JA, Dulai PS, et al. No benefit of concomitant 5-aminosalicylates in patients with ulcerative colitis escalated to biologic therapy: pooled analysis of individual participant data from clinical trials. Am J Gastroenterol. 2018; 113:1197–1205.
Article75. Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003; 348:601–608.
Article76. Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004; 2:542–553.
Article77. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010; 59:49–54.
Article78. Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. 2011; 60:41–48.
Article79. Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn’s disease. Am J Gastroenterol. 2008; 103:944–948.
Article80. Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013; 108:40–47.
Article81. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014; 63:919–927.
Article82. Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013; 19:2568–2576.
Article83. Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014; 12:80–84.
Article84. Yanai H, Lichtenstein L, Assa A, et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015; 13:522–530.
Article85. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010; 105:1133–1139.
Article86. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015; 148:1320–1329.
Article87. D’Haens G, Vermeire S, Lambrecht G, et al. Increasing infliximab dose based on symptoms, biomarkers, and serum drug concentrations does not increase clinical, endoscopic, and corticosteroid-free remission in patients with active luminal Crohn’s disease. Gastroenterology. 2018; 154:1343–1351.
Article88. Filippi J, Laharie D, Michiels C, et al. Efficacy of sustained combination therapy for at least 6 months with thiopurines and infliximab in patients with ulcerative colitis in clinical remission: a retrospective multicenter French experience. J Crohns Colitis. 2015; 9:252–258.
Article89. Targownik LE, Benchimol EI, Bernstein CN, et al. Combined biologic and immunomodulatory therapy is superior to monotherapy for decreasing the risk of inflammatory bowel disease-related complications. J Crohns Colitis. 2020; 14:1354–1363.
Article90. Kobayashi T, Udagawa E, Uda A, Hibi T, Hisamatsu T. Impact of immunomodulator use on treatment persistence in patients with ulcerative colitis: a claims database analysis. J Gastroenterol Hepatol. 2020; 35:225–232.
Article91. Shin SY, Park SJ, Kim Y, et al. Clinical outcomes and predictors of response for adalimumab in patients with moderately to severely active ulcerative colitis: a KASID prospective multicenter cohort study. Intest Res. 2022; 20:350–360.
Article92. Feagan BG, Schreiber S, Wolf DC, et al. Sustained clinical remission with vedolizumab in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2019; 25:1028–1035.
Article93. Perry C, Fischer K, Elmoursi A, et al. Vedolizumab dose escalation improves therapeutic response in a subset of patients with ulcerative colitis. Dig Dis Sci. 2021; 66:2051–2058.
Article94. Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol. 2017; 15:1750–1757.
Article95. Samaan MA, Pavlidis P, Johnston E, et al. Vedolizumab: early experience and medium-term outcomes from two UK tertiary IBD centres. Frontline Gastroenterol. 2017; 8:196–202.
Article96. Shmidt E, Kochhar G, Hartke J, et al. Predictors and management of loss of response to vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis. 2018; 24:2461–2467.
Article97. Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017; 11:400–411.
Article98. Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:838–846.
Article99. Yzet C, Diouf M, Singh S, et al. No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: a meta-analysis. Clin Gastroenterol Hepatol. 2021; 19:668–679.
Article100. Ng SC, Hilmi IN, Blake A, et al. Low frequency of opportunistic infections in patients receiving vedolizumab in clinical trials and post-marketing setting. Inflamm Bowel Dis. 2018; 24:2431–2441.
Article101. Banerjee R, Ali R, Wei SC, Adsul S. Biologics for the management of inflammatory bowel disease: a review in tuberculosis-endemic countries. Gut Liver. 2020; 14:685–698.
Article102. Adedokun OJ, Xu Z, Marano C, et al. Ustekinumab pharmacokinetics and exposure response in a phase 3 randomized trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020; 18:2244–2255.
Article103. Xu Y, Hu C, Chen Y, et al. Population pharmacokinetics and exposure-response modeling analyses of ustekinumab in adults with moderately to severely active ulcerative colitis. J Clin Pharmacol. 2020; 60:889–902.
Article104. Hu A, Kotze PG, Burgevin A, et al. Combination therapy does not improve rate of clinical or endoscopic remission in patients with inflammatory bowel diseases treated with vedolizumab or ustekinumab. Clin Gastroenterol Hepatol. 2021; 19:1366–1376.
Article105. Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: realworld experience from a multicenter cohort study. Inflamm Bowel Dis. 2017; 23:833–839.
Article106. US Food and Drug Administration. XELJANZ® (tofacitinib): highlights of prescribing information [Internet]. c2009 [cited 2021 Feb 8]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203214s024,208246s010lbl.pdf.107. European Medicines Agency. Xeljanz (tofacitinib): summary of product characteristics [Internet]. c2017 [cited 2021 Feb 8]. https://www.ema.europa.eu/en/glossary/summary-product-characteristics.108. Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020; 51:271–280.
Article109. Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020; 14:1385–1393.110. Sandborn WJ, Panés J, D’Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol. 2019; 17:1541–1550.
Article111. de Mora F. Biosimilar: what it is not. Br J Clin Pharmacol. 2015; 80:949–956.
Article112. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013; 72:1613–1620.
Article113. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013; 72:1605–1612.
Article114. Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016; 18:82.
Article115. Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016; 18:25.
Article116. Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomised, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017; 76:58–64.
Article117. Smolen JS, Choe JY, Prodanovic N, et al. Comparing biosimilar SB2 with reference infliximab after 54 weeks of a doubleblind trial: clinical, structural and safety results. Rheumatology (Oxford). 2017; 56:1771–1779.
Article118. Smolen JS, Choe JY, Prodanovic N, et al. Safety, immunogenicity and efficacy after switching from reference infliximab to biosimilar SB2 compared with continuing reference infliximab and SB2 in patients with rheumatoid arthritis: results of a randomised, double-blind, phase III transition study. Ann Rheum Dis. 2018; 77:234–240.
Article119. Cohen SB, Alten R, Kameda H, et al. A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy. Arthritis Res Ther. 2018; 20:155.
Article120. Genovese MC, Sanchez-Burson J, Oh M, et al. Comparative clinical efficacy and safety of the proposed biosimilar ABP 710 with infliximab reference product in patients with rheumatoid arthritis. Arthritis Res Ther. 2020; 22:60.
Article121. Jani RH, Gupta R, Bhatia G, et al. A prospective, randomized, double-blind, multicentre, parallel-group, active controlled study to compare efficacy and safety of biosimilar adalimumab (Exemptia; ZRC-3197) and adalimumab (Humira) in patients with rheumatoid arthritis. Int J Rheum Dis. 2016; 19:1157–1168.
Article122. Cohen S, Genovese MC, Choy E, et al. Efficacy and safety of the biosimilar ABP 501 compared with adalimumab in patients with moderate to severe rheumatoid arthritis: a randomised, double-blind, phase III equivalence study. Ann Rheum Dis. 2017; 76:1679–1687.
Article123. Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017; 76:1093–1102.
Article124. Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis. 2018; 77:914–921.
Article125. Blauvelt A, Lacour JP, Fowler JF Jr, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018; 179:623–631.
Article126. Wiland P, Jeka S, Dokoupilová E, et al. Switching to biosimilar SDZ-ADL in patients with moderate-to-severe active rheumatoid arthritis: 48-week efficacy, safety and immunogenicity results from the phase III, randomized, double-blind ADMYRA study. BioDrugs. 2020; 34:809–823.
Article127. Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol. 2018; 70:40–48.128. Weinblatt ME, Baranauskaite A, Dokoupilova E, et al. Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol. 2018; 70:832–840.
Article129. Fleischmann RM, Alten R, Pileckyte M, et al. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res Ther. 2018; 20:178.130. Genovese MC, Glover J, Greenwald M, et al. FKB327, an adalimumab biosimilar, versus the reference product: results of a randomized, phase III, double-blind study, and its openlabel extension. Arthritis Res Ther. 2019; 21:281.
Article131. Gecse KB, Lovász BD, Farkas K, et al. Efficacy and safety of the biosimilar infliximab CT-P13 treatment in inflammatory bowel diseases: a prospective, multicentre, nationwide cohort. J Crohns Colitis. 2016; 10:133–140.
Article132. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017; 23:233–243.133. Armuzzi A, Fiorino G, Variola A, et al. The PROSIT cohort of infliximab biosimilar in IBD: a prolonged follow-up on the effectiveness and safety across Italy. Inflamm Bowel Dis. 2019; 25:568–579.
Article134. Ebada MA, Elmatboly AM, Ali AS, et al. An updated systematic review and meta-analysis about the safety and efficacy of infliximab biosimilar, CT-P13, for patients with inflammatory bowel disease. Int J Colorectal Dis. 2019; 34:1633–1652.
Article135. Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019; 393:1699–1707.
Article136. Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CTP13 maintenance in inflammatory bowel disease. Gastroenterology. 2021; 160:2340–2353.
Article137. Hanauer S, Liedert B, Balser S, Brockstedt E, Moschetti V, Schreiber S. Safety and efficacy of BI 695501 versus adalimumab reference product in patients with advanced Crohn’s disease (VOLTAIRE-CD): a multicentre, randomised, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2021; 6:816–825.
Article138. Chandra A, Kanth R, Thareja S. Efficacy and safety of adalimumab biosimilar (Exemptia) in moderate-to-severe steroidrefractory ulcerative colitis patients: real-life outcomes in resource-constrained setting at 24-weeks follow-up. Biologics. 2019; 13:191–200.139. Kamat N, Kedia S, Ghoshal UC, et al. Effectiveness and safety of adalimumab biosimilar in inflammatory bowel disease: a multicenter study. Indian J Gastroenterol. 2019; 38:44–54.
Article140. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017; 389:2304–2316.141. Goll GL, Jørgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: open-label extension of the NOR-SWITCH trial. J Intern Med. 2019; 285:653–669.142. Jørgensen KK, Goll GL, Sexton J, et al. Efficacy and safety of CT-P13 in inflammatory bowel disease after switching from originator infliximab: exploratory analyses from the NORSWITCH main and extension trials. BioDrugs. 2020; 34:681–694.
Article143. Bergqvist V, Kadivar M, Molin D, et al. Switching from originator infliximab to the biosimilar CT-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol. 2018; 11:1756284818801244.
Article144. Cingolani L, Barberio B, Zingone F, et al. Adalimumab biosimilars, ABP501 and SB5, are equally effective and safe as adalimumab originator. Sci Rep. 2021; 11:10368.
Article145. Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005; 128:1805–1811.
Article146. Gustavsson A, Järnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis. 3-Year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010; 32:984–989.
Article147. Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012; 380:1909–1915.
Article148. Kim EH, Kim DH, Park SJ, et al. Infliximab versus cyclosporine treatment for severe corticosteroid-refractory ulcerative colitis: a Korean, retrospective, single center study. Gut Liver. 2015; 9:601–606.
Article149. Song EM, Oh EH, Hwang SW, et al. Comparison of outcomes of cyclosporine A and infliximab for steroid-refractory acute severe ulcerative colitis. J Gastroenterol Hepatol. 2021; 36:2463–2470.
Article150. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016; 1:15–24.
Article151. Christensen B, Gibson PR, Micic D, et al. Safety and efficacy of combination treatment with calcineurin inhibitors and vedolizumab in patients with refractory inflammatory bowel disease. Clin Gastroenterol Hepatol. 2019; 17:486–493.
Article152. Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:502–509.
Article153. Gibson DJ, Doherty J, McNally M, et al. Comparison of medium to long-term outcomes of acute severe ulcerative colitis patients receiving accelerated and standard infliximab induction. Frontline Gastroenterol. 2019; 11:441–447.
Article154. Sebastian S, Myers S, Argyriou K, et al. Infliximab induction regimens in steroid-refractory acute severe colitis: a multicentre retrospective cohort study with propensity score analysis. Aliment Pharmacol Ther. 2019; 50:675–683.
Article155. Shah SC, Naymagon S, Panchal HJ, Sands BE, Cohen BL, Dubinsky MC. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018; 24:651–659.
Article156. Honap S, Pavlidis P, Ray S, et al. Tofacitinib in acute severe ulcerative colitis: a real-world tertiary center experience. Inflamm Bowel Dis. 2020; 26:e147–e149.157. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis. 2020; 14:1026–1028.
Article158. Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2019; 17:988–990.
Article159. Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2019; 17:139–147.
Article160. Uzzan M, Bresteau C, Laharie D, et al. Tofacitinib as salvage therapy for 55 patients hospitalised with refractory severe ulcerative colitis: a GETAID cohort. Aliment Pharmacol Ther. 2021; 54:312–319.
Article161. Lau C, Dubinsky M, Melmed G, et al. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg. 2015; 261:487–496.
Article162. Zittan E, Milgrom R, Ma GW, et al. Preoperative anti-tumor necrosis factor therapy in patients with ulcerative colitis is not associated with an increased risk of infectious and noninfectious complications after ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2016; 22:2442–2447.
Article163. Ward ST, Mytton J, Henderson L, et al. Anti-TNF therapy is not associated with an increased risk of post-colectomy complications, a population-based study. Colorectal Dis. 2018; 20:416–423.
Article164. Novello M, Stocchi L, Steele SR, et al. Case-matched comparison of postoperative outcomes following surgery for inflammatory bowel disease after exposure to vedolizumab vs other biologics. J Crohns Colitis. 2020; 14:185–191.
Article165. Abelson JS, Michelassi F, Mao J, Sedrakyan A, Yeo H. Higher surgical morbidity for ulcerative colitis patients in the era of biologics. Ann Surg. 2018; 268:311–317.
Article166. Yang Z, Wu Q, Wu K, Fan D. Meta-analysis: pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2010; 31:486–492.
Article167. Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis. 2013; 7:853–867.
Article168. El-Hussuna A, Qvist N, Zangenberg MS, et al. No effect of anti-TNF-α agents on the surgical stress response in patients with inflammatory bowel disease undergoing bowel resections: a prospective multi-center pilot study. BMC Surg. 2018; 18:91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advancements in the Management of Moderate-to-Severe Ulcerative Colitis: A Revised 2023 Korean Treatment Guidelines
- Current and Emerging Biologics for Ulcerative Colitis
- Safety of Biologics and Small Molecules for Inflammatory Bowel Diseases in Organ Transplant Recipients
- Old and New Biologics and Small Molecules in Inflammatory Bowel Disease: Anti-Tumor Necrosis Factors
- Therapeutic Drug Monitoring of Biologics for Patients with Inflammatory Bowel Diseases: How, When, and for Whom?