Diabetes Metab J.
2023 Jan;47(1):72-81. 10.4093/dmj.2022.0035.
Performance of Fast-Acting Aspart Insulin as Compared to Aspart Insulin in Insulin Pump for Managing Type 1 Diabetes Mellitus: A Meta-Analysis
- Affiliations
-
- 1Department of Endocrinology, Center For Endocrinology Diabetes Arthritis & Rheumatism (CEDAR) Superspeciality Healthcare, New Delhi, India
- 2Department of Medicine, Postgraduate Institute of Medical Education and Research, Chandigarh, India
- 3Department of Cardiology, Holy Heart Advanced Cardiac Care Center, Rohtak, India
- 4Department of Rheumatology, CEDAR Superspeciality Healthcare, New Delhi, India
- KMID: 2538932
- DOI: http://doi.org/10.4093/dmj.2022.0035
Abstract
- Background
No meta-analysis has analysed efficacy and safety of fast-acting aspart insulin (FIAsp) with insulin pump in type 1 diabetes mellitus (T1DM).
Methods
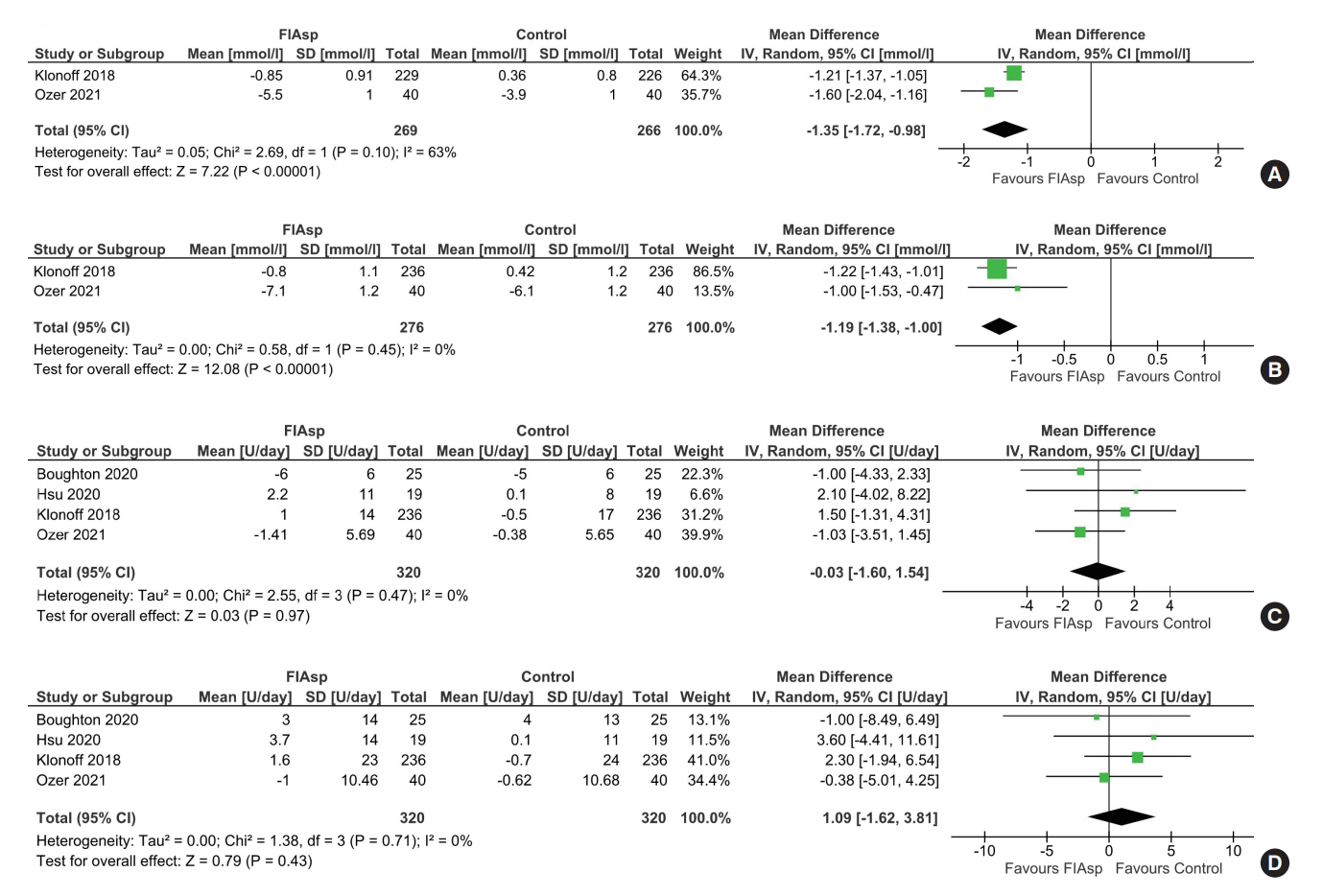
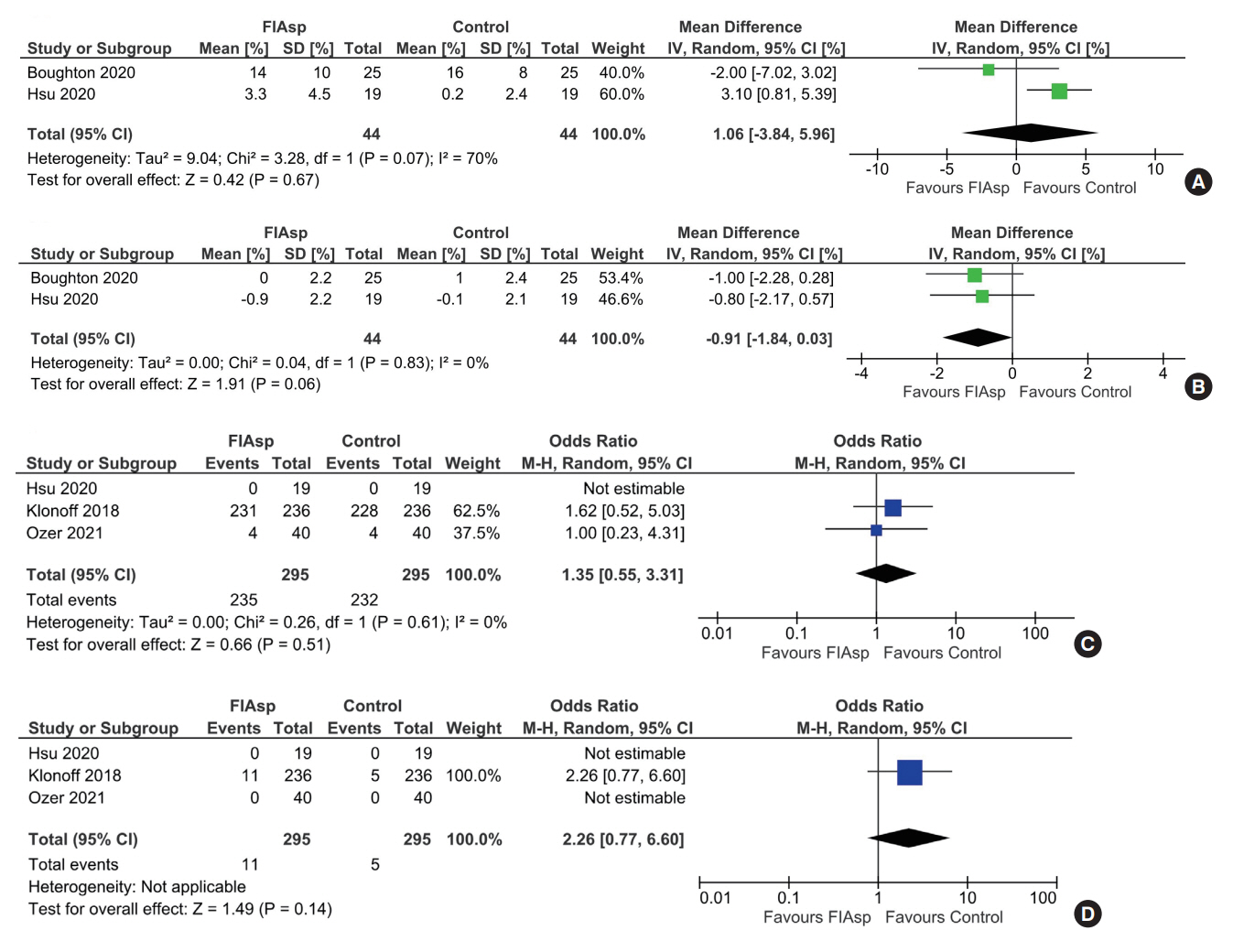
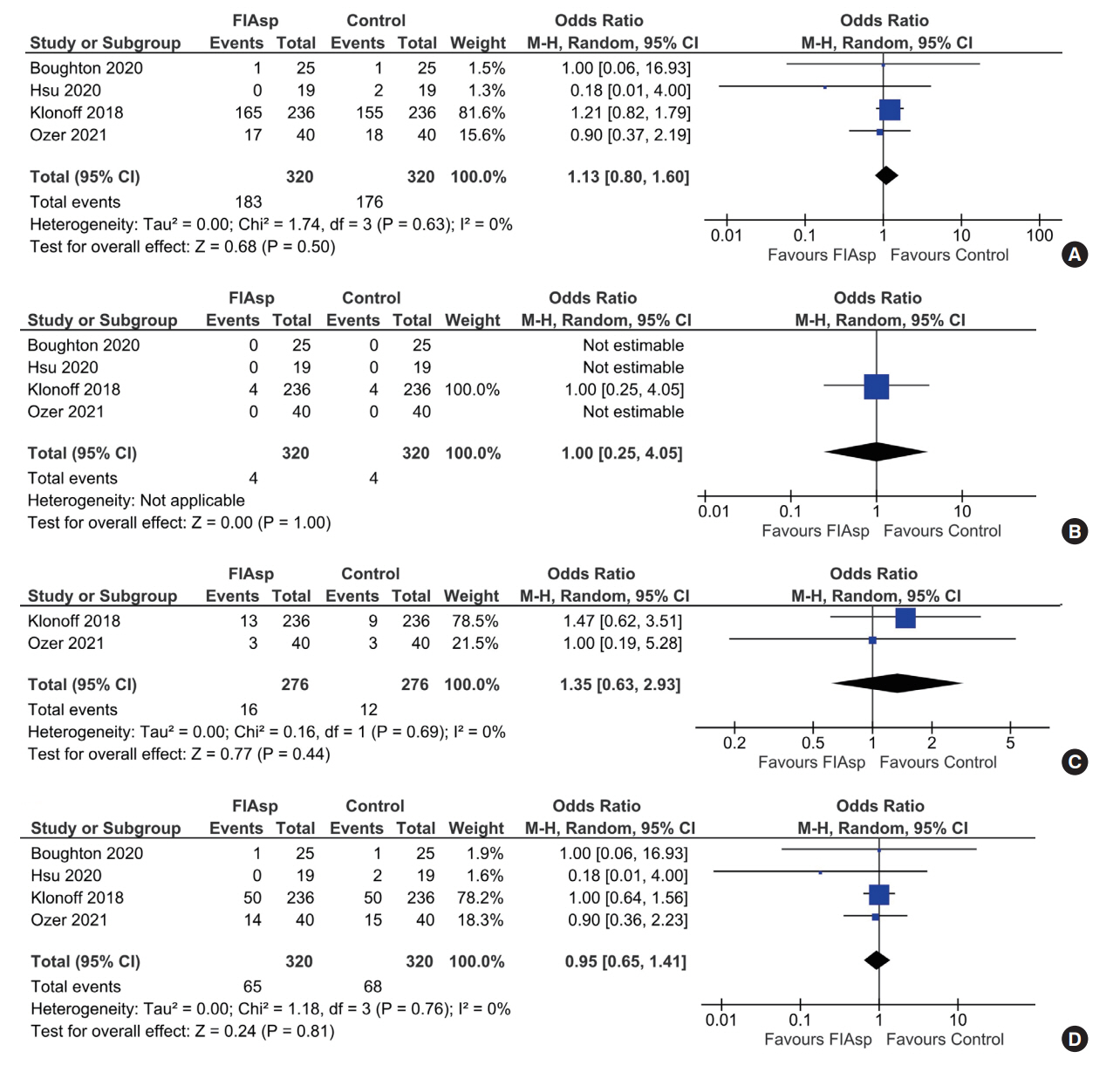
Electronic databases were searched for randomised controlled trials (RCTs) involving T1DM patients on insulin pump receiving FIAsp in intervention arm, and placebo/active comparator insulin in control arm. Primary outcome was to evaluate changes in 1- and 2-hour post-prandial glucose (1hPPG and 2hPPG). Secondary outcomes were to evaluate alterations in percentage time with blood glucose <3.9 mmol/L (hypoglycaemia), time in range (TIR) blood glucose 3.9 to 10 mmol/L, insulin requirements and adverse events.
Results
Data from four RCTs involving 640 patients was analysed. FIAsp use in insulin pump was associated with significantly greater lowering of 1hPPG (mean difference [MD], –1.35 mmol/L; 95% confidence interval [CI], –1.72 to –0.98; P<0.01; I2=63%) and 2hPPG (MD, –1.19 mmol/L; 95% CI, –1.38 to –1.00; P<0.01; I2=0%) as compared to controls. TIR was comparable among groups (MD, 1.06%; 95% CI, –3.84 to 5.96; P=0.67; I2=70%). Duration of blood glucose <3.9 mmol/L was lower in FIAsp group, approaching significance (MD, –0.91%; 95% CI, –1.84 to 0.03; P=0.06; I2=0%). Total hypoglycaemic episodes (risk ratio [RR], 1.35; 95% CI, 0.55 to 3.31; P=0.51; I2=0%), severe hypoglycaemia (RR, 2.26; 95% CI, 0.77 to 6.66; P=0.14), infusion site reactions (RR, 1.35; 95% CI, 0.63 to 2.93; P=0.77; I2=0%), and treatment-emergent adverse events (RR, 1.13; 95% CI, 0.80 to 1.60; P=0.50; I2=0%) were comparable.
Conclusion
FIAsp use in insulin pump is associated with better post-prandial glycaemic control with no increased hypoglycaemia or glycaemic variability.
Figure
Reference
-
1. Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017; 19:155–63.
Article2. Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017; 56:551–9.
Article3. Heise T, Hovelmann U, Brondsted L, Adrian CL, Nosek L, Haahr H. Faster-acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015; 17:682–8.
Article4. Haahr H, Heise T. Fast-acting insulin aspart: a review of its pharmacokinetic and pharmacodynamic properties and the clinical consequences. Clin Pharmacokinet. 2020; 59:155–72.
Article5. Ozer K, Cooper AM, Ahn LP, Waggonner CR, Blevins TC. Fast acting insulin aspart compared with insulin aspart in the Medtronic 670G hybrid closed loop system in type 1 diabetes: an open label crossover study. Diabetes Technol Ther. 2021; 23:286–92.
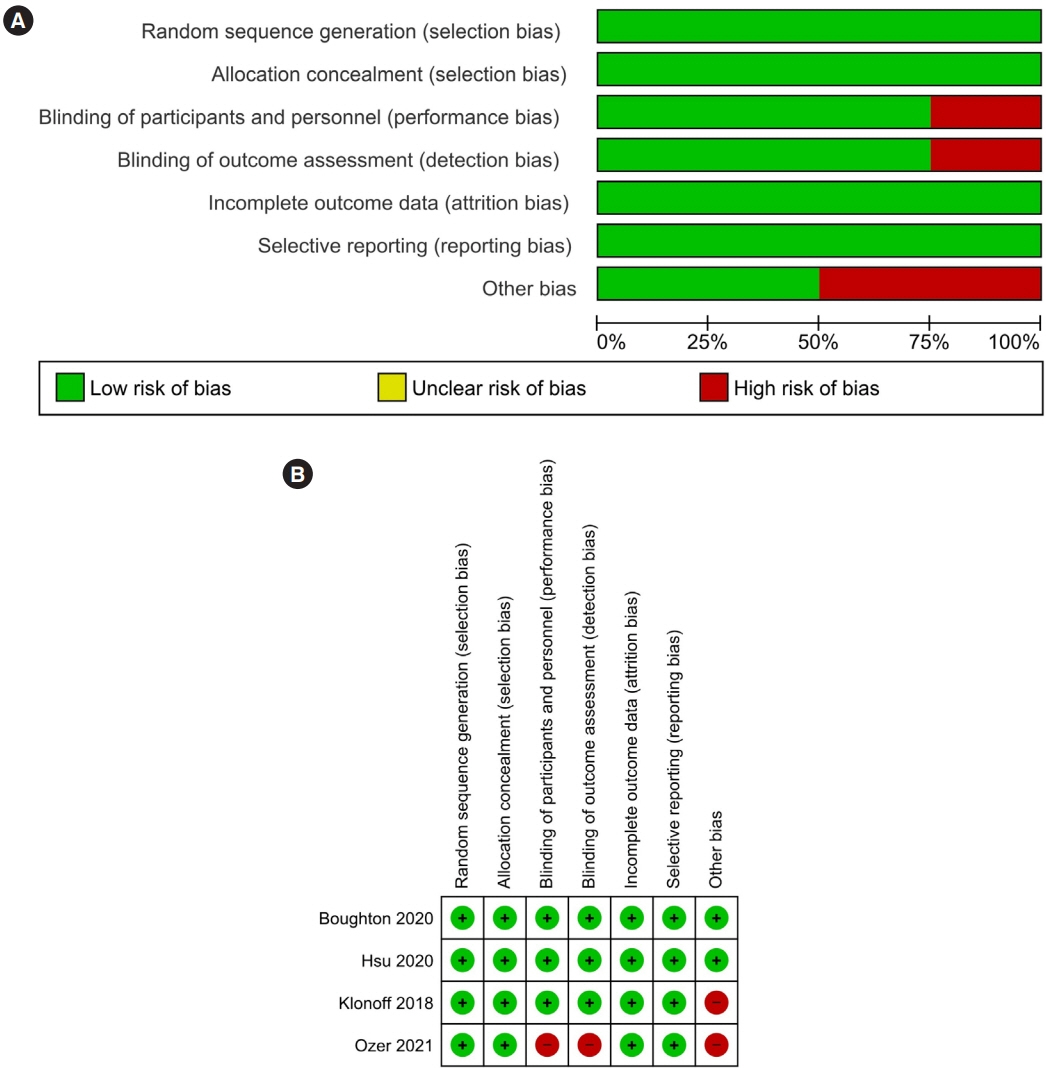
Article6. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article7. Dutta D, Bhattacharya S, Surana V, Aggarwal S, Singla R, Khandelwal D, et al. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: a meta-analysis. Diabetes Metab Syndr. 2020; 14:1759–68.
Article8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
Article9. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–6.
Article10. Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000; 4:1–115.
Article11. Boughton CK, Hartnell S, Thabit H, Poettler T, Herzig D, Wilinska ME, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: a double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. 2021; 23:1389–96.
Article12. Klonoff DC, Evans ML, Lane W, Kempe HP, Renard E, DeVries JH, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab. 2019; 21:961–7.
Article13. Hsu L, Buckingham B, Basina M, Ekhlaspour L, von Eyben R, Wang J, et al. Fast-acting insulin aspart use with the MiniMedTM 670G System. Diabetes Technol Ther. 2021; 23:1–7.14. Russell SJ, Balliro C, Ekelund M, El-Khatib F, Graungaard T, Greaux E, et al. Improvements in glycemic control achieved by altering the tmax setting in the iLet® bionic pancreas when using fast-acting insulin aspart: a randomized trial. Diabetes Ther. 2021; 12:2019–33.
Article15. Grosman B, Wu D, Parikh N, Roy A, Voskanyan G, Kurtz N, et al. Fast-acting insulin aspart (Fiasp®) improves glycemic outcomes when used with MiniMedTM 670G hybrid closed-loop system in simulated trials compared to NovoLog®. Comput Methods Programs Biomed. 2021; 205:106087.16. Tsoukas MA, Cohen E, Legault L, von Oettingen JE, Yale JF, Vallis M, et al. Alleviating carbohydrate counting with a FiASP-plus-pramlintide closed-loop delivery system (artificial pancreas): feasibility and pilot studies. Diabetes Obes Metab. 2021; 23:2090–8.
Article17. Avgerinos I, Papanastasiou G, Karagiannis T, Michailidis T, Liakos A, Mainou M, et al. Ultra-rapid-acting insulins for adults with diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2021; 23:2395–401.
Article18. Rys P, Pankiewicz O, Lach K, Kwaskowski A, Skrzekowska-Baran I, Malecki MT. Efficacy and safety comparison of rapidacting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: a systematic review. Diabetes Metab. 2011; 37:190–200.
Article19. Evans M, Wilkinson M, Giannpolou A. Fast-acting insulin aspart: the rationale for a new mealtime insulin. Diabetes Ther. 2019; 10:1793–800.
Article20. Evans M, Ceriello A, Danne T, De Block C, DeVries JH, Lind M, et al. Use of fast-acting insulin aspart in insulin pump therapy in clinical practice. Diabetes Obes Metab. 2019; 21:2039–47.
Article21. 1MG: FIAsp insulin online purchase. Available from: https://www.1mg.com/drugs/fiasp-100iu-ml-penfill-506091 (cited 2022 Feb 14).22. 1MG: Novorapid insulin online purchase. Available from: https://www.1mg.com/drugs/novorapid-100iu-ml-solution-for-injection-372477 (cited 2022 Feb 14).23. 1MG: Aspart insulin online purchase. Available from: https://www.1mg.com/drugs/humalog-100iu-ml-solution-for-injection-341834 (cited 2022 Feb 14).24. Lee B. How much does insulin cost? Here’s how 28 brands and generics compare. Available from: https://www.goodrx.com/healthcare-access/research/how-much-does-insulin-cost-compare-brands (updated 2022 Jan 26).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Favorable Glycemic Control with Once-Daily Insulin Degludec/Insulin Aspart after Changing from Basal Insulin in Adults with Type 2 Diabetes (Endocrinol Metab 2019; 34:382-9, Han Na Jang et al.)
- Effective Use of Insulin Pump in Patients with Type 1 Diabetes
- Medical Therapy in Pregnant Women with Diabetes
- Response: Favorable Glycemic Control with Once-Daily Insulin Degludec/Insulin Aspart after Changing from Basal Insulin in Adults with Type 2 Diabetes (Endocrinol Metab 2019; 34:382-9, Han Na Jang et al.)
- Comparision of Body Image between DM Patients who used Insulin Pump and didn't use Insulin Pump