Lab Med Online.
2022 Oct;12(4):244-261. 10.47429/lmo.2022.12.4.244.
Recommendations for Clinical Application of Pharmacogenetic Test Results Interpretation by Clinical Laboratories
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea
- 2Department of Laboratory Medicine, Soonchunhyang University Seoul Hospital, Seoul, Korea
- 3Department of Laboratory Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- KMID: 2538612
- DOI: http://doi.org/10.47429/lmo.2022.12.4.244
Abstract
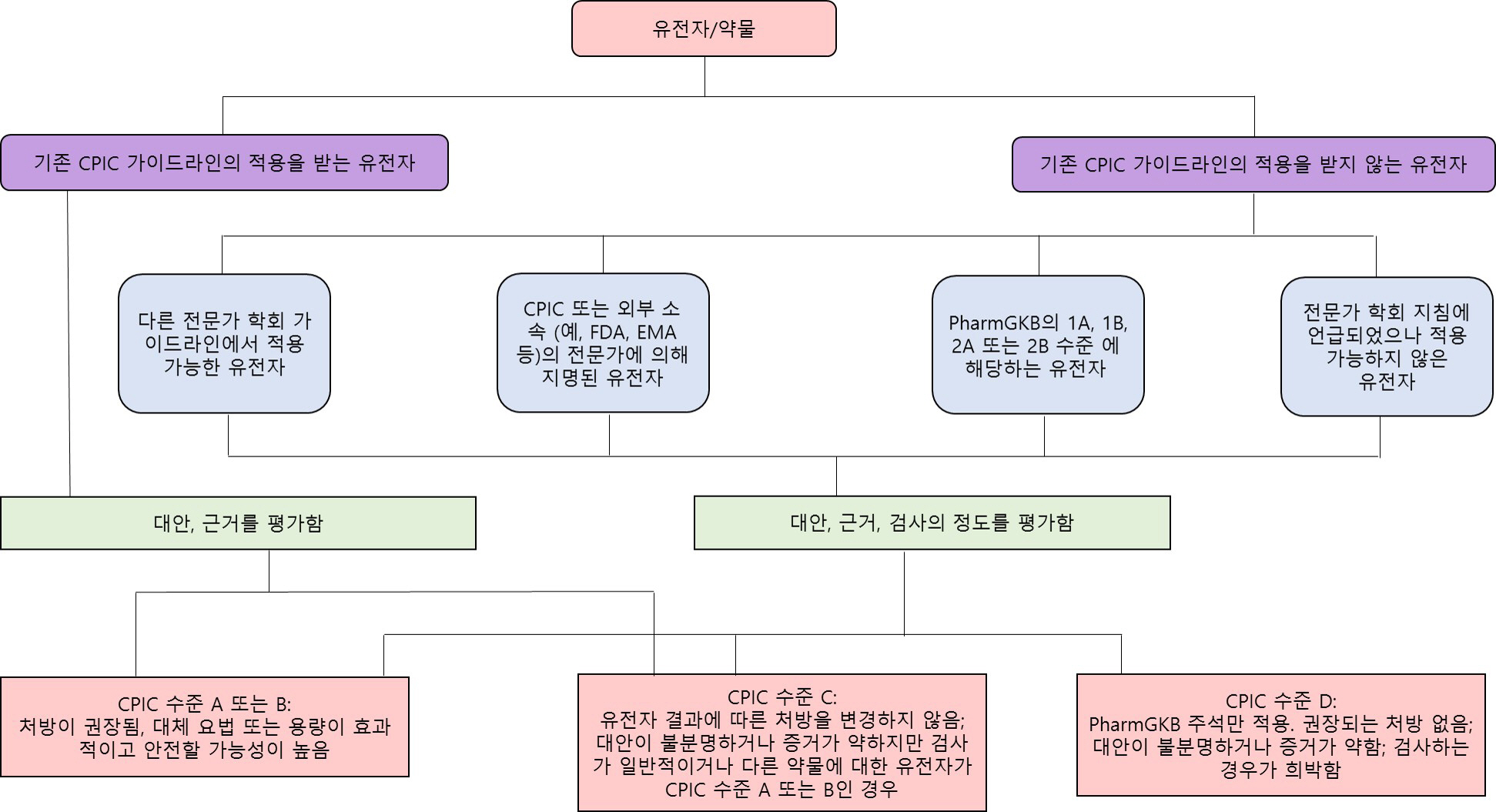
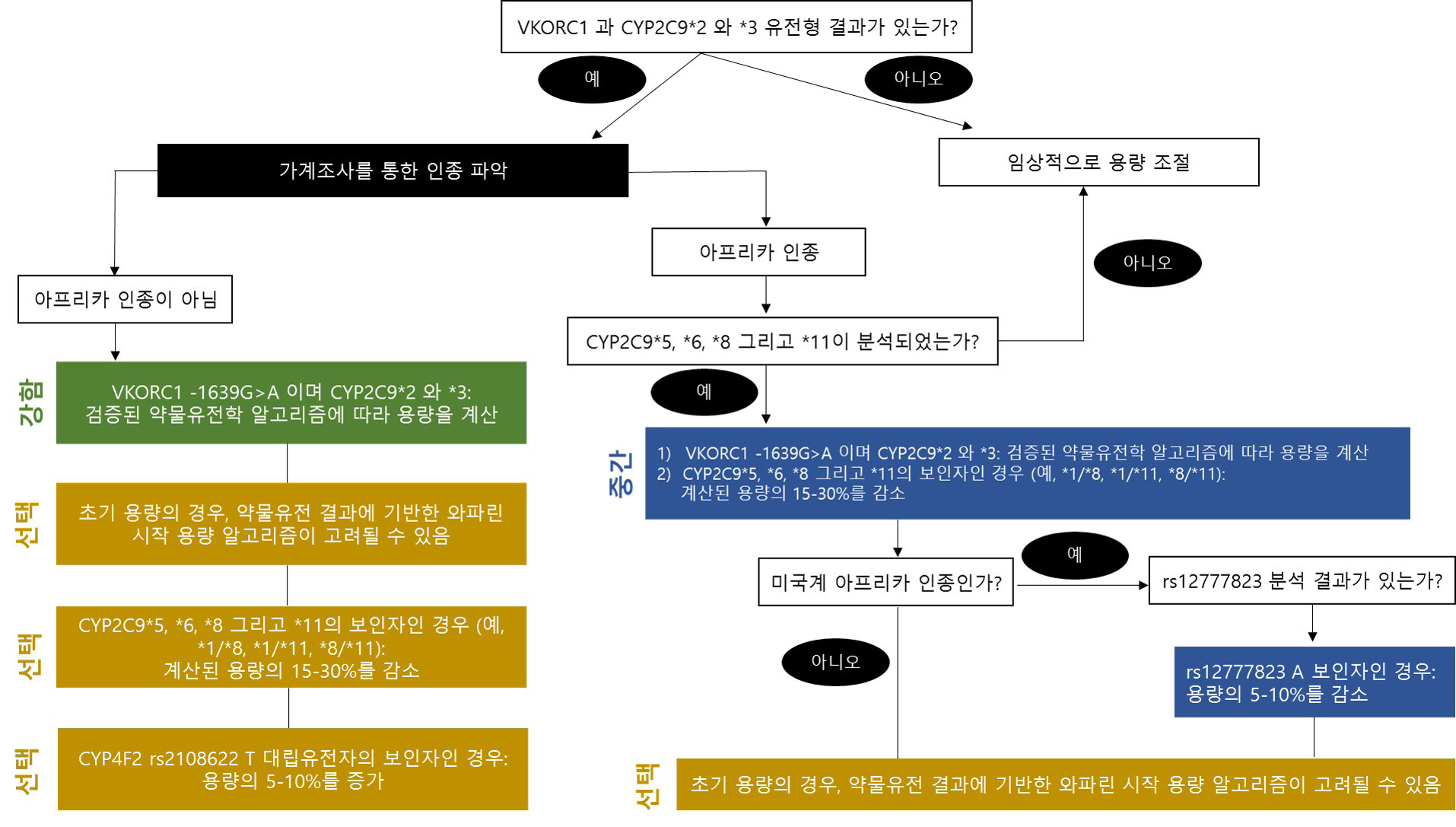
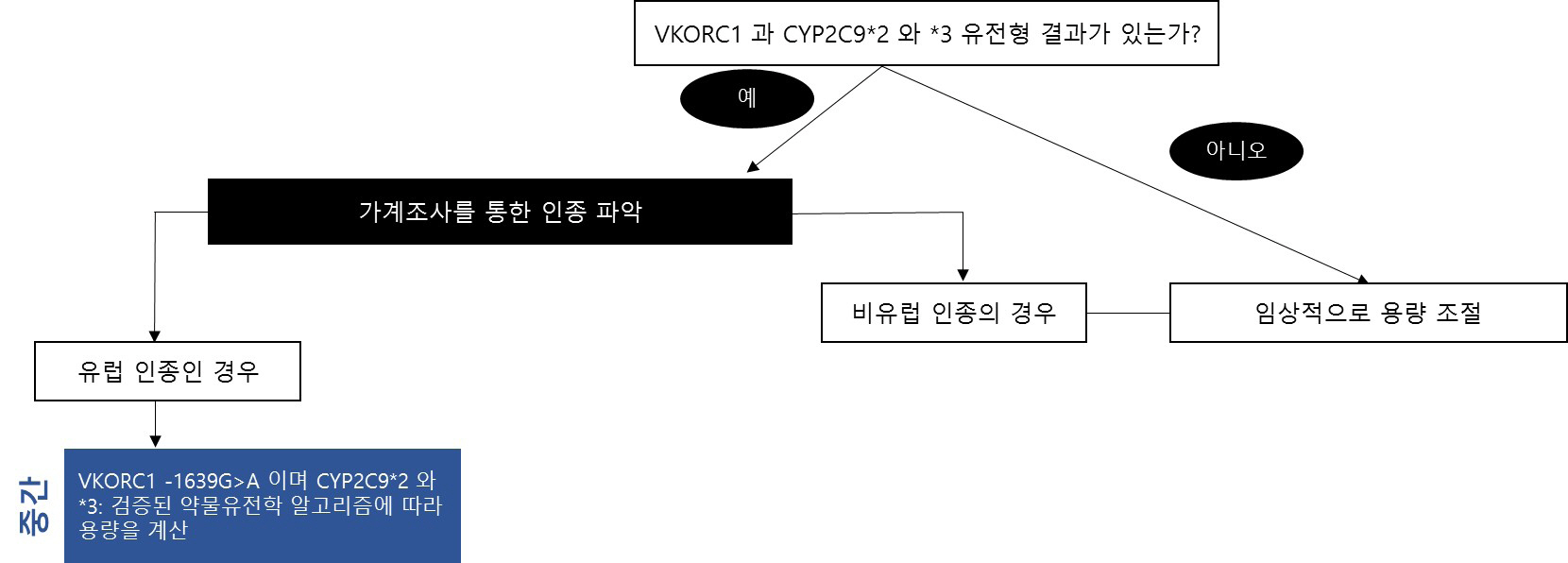
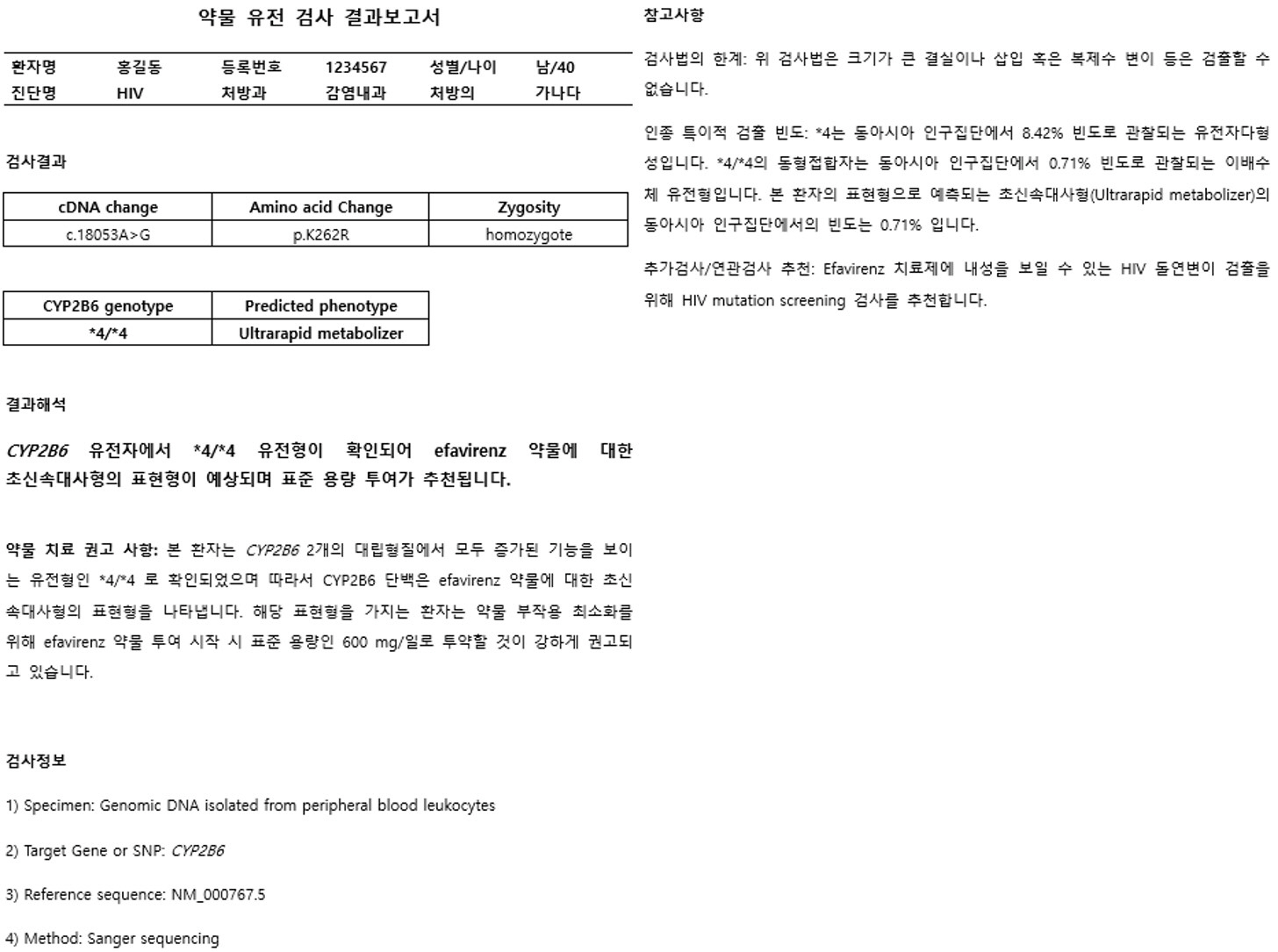
- As various clinical genetic tests become widely available, the quality of clinical practice in several medical fields, including clinical pharmacogenetics, can be dramatically improved based on genetic test results. Importantly, standardization and the appropriate result interpretation of pharmacogenetic tests are crucial steps toward successful clinical implementation. Among various guidelines published to support accurate clinical decisions, the most commonly utilized evidence is systematically organized in the Clinical Pharmacogenetics Implementation Consortium guidelines, which are regularly updated. The establishment of accurate evidence for each drug-gene pair in pharmacogenetic test result interpretation is essential, but is a complicated and challenging issue. Herein, we provide up-to-date evidence for preparing professional interpretative comments in clinical pharmacogenetic test reports. We provide therapeutic recommendations based on pharmacological genotype-phenotype annotations for a total of 32 drug-gene pairs with sufficient clinical evidence. This document aims to provide reliable evidence and practical information for pharmacogenetic variant interpretation in routine clinical settings, to maximize the efficacy and minimize the side-effects of various pharmacotherapies.
Figure
Reference
-
1. Abdullah-Koolmees H, van Keulen AM, Nijenhuis M, Deneer VHM. 2021; Pharmacogenetics Guidelines: Overview and Comparison of the DPWG, CPIC, CPNDS, and RNPGx Guidelines. Front Pharmacol. 11:595219. DOI: 10.3389/fphar.2020.595219. PMID: 33568995. PMCID: PMC7868558.
Article2. Kim S, Yun YM, Kim IS, Song SH, Woo HI, Lee KA, et al. 2016; Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines Part 1. Lab Med Online. 6:119–33. DOI: 10.3343/lmo.2016.6.3.119.
Article3. Kim S, Yun YM, Kim IS, Song SH, Woo HI, Lee KA, et al. 2016; Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines Part 2. Lab Med Online. 6:193–213. DOI: 10.3343/lmo.2016.6.4.193.
Article4. Esposito D, Weile J, Shendure J, Starita LM, Papenfuss AT, Roth FP, et al. 2019; MaveDB: an open-source platform to distribute and interpret data from multiplexed assays of variant effect. Genome Biol. 20:223. DOI: 10.1186/s13059-019-1845-6. PMID: 31679514. PMCID: PMC6827219.
Article5. Zhang L, Sarangi V, Moon I, Yu J, Liu D, Devarajan S, et al. 2020; CYP2C9 and CYP2C19: Deep Mutational Scanning and Functional Characterization of Genomic Missense Variants. Clin Transl Sci. 13:727–42. DOI: 10.1111/cts.12758. PMID: 32004414. PMCID: PMC7359949.
Article6. Desta Z, Gammal RS, Gong L, Whirl-Carrillo M, Gaur AH, Sukasem C, et al. 2019; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and Efavirenz-Containing Antiretroviral Therapy. Clin Pharmacol Ther. 106:726–33. DOI: 10.1002/cpt.1477. PMID: 31006110. PMCID: PMC6739160.7. Scott SA, Sangkuhl K, Stein CM, Hulot JS, Mega JL, Roden DM, et al. 2013; Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 94:317–23. DOI: 10.1038/clpt.2013.105. PMID: 23698643. PMCID: PMC3748366.
Article8. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. 2019; Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin Pharmacol Ther. 105:1095–105. DOI: 10.1002/cpt.1304. PMID: 30447069. PMCID: PMC6576267.9. Ramsey LB, Johnson SG, Caudle KE, Haidar CE, Voora D, Wilke RA, et al. 2014; The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 96:423–8. DOI: 10.1038/clpt.2014.125. PMID: 24918167. PMCID: PMC4169720.
Article10. Gammal RS, Court MH, Haidar CE, Iwuchukwu OF, Gaur AH, Alvarellos M, et al. 2016; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clin Pharmacol Ther. 99:363–9. DOI: 10.1002/cpt.269. PMID: 26417955. PMCID: PMC4785051.11. Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Müller DJ, Shimoda K, et al. 2017; Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 102:37–44. DOI: 10.1002/cpt.597. PMID: 27997040. PMCID: PMC5478479.
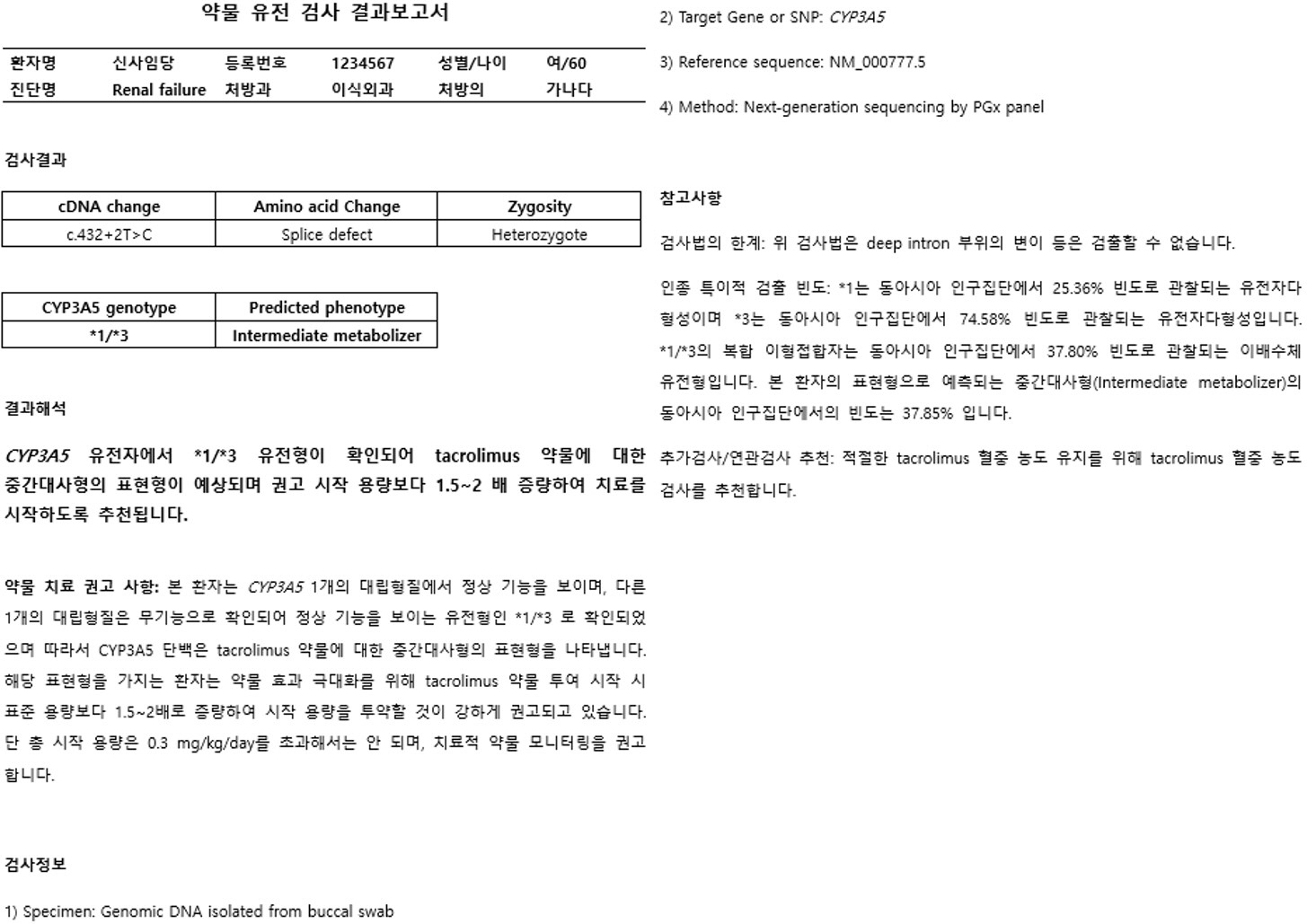
Article12. Hicks JK, Bishop JR, Sangkuhl K, Müller DJ, Ji Y, Leckband SG, et al. 2015; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther. 98:127–34. DOI: 10.1002/cpt.147. PMID: 25974703. PMCID: PMC4512908.13. Birdwell KA, Decker B, Barbarino JM, Peterson JF, Stein CM, Sadee W, et al. 2015; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 98:19–24. DOI: 10.1002/cpt.113. PMID: 25801146. PMCID: PMC4481158.
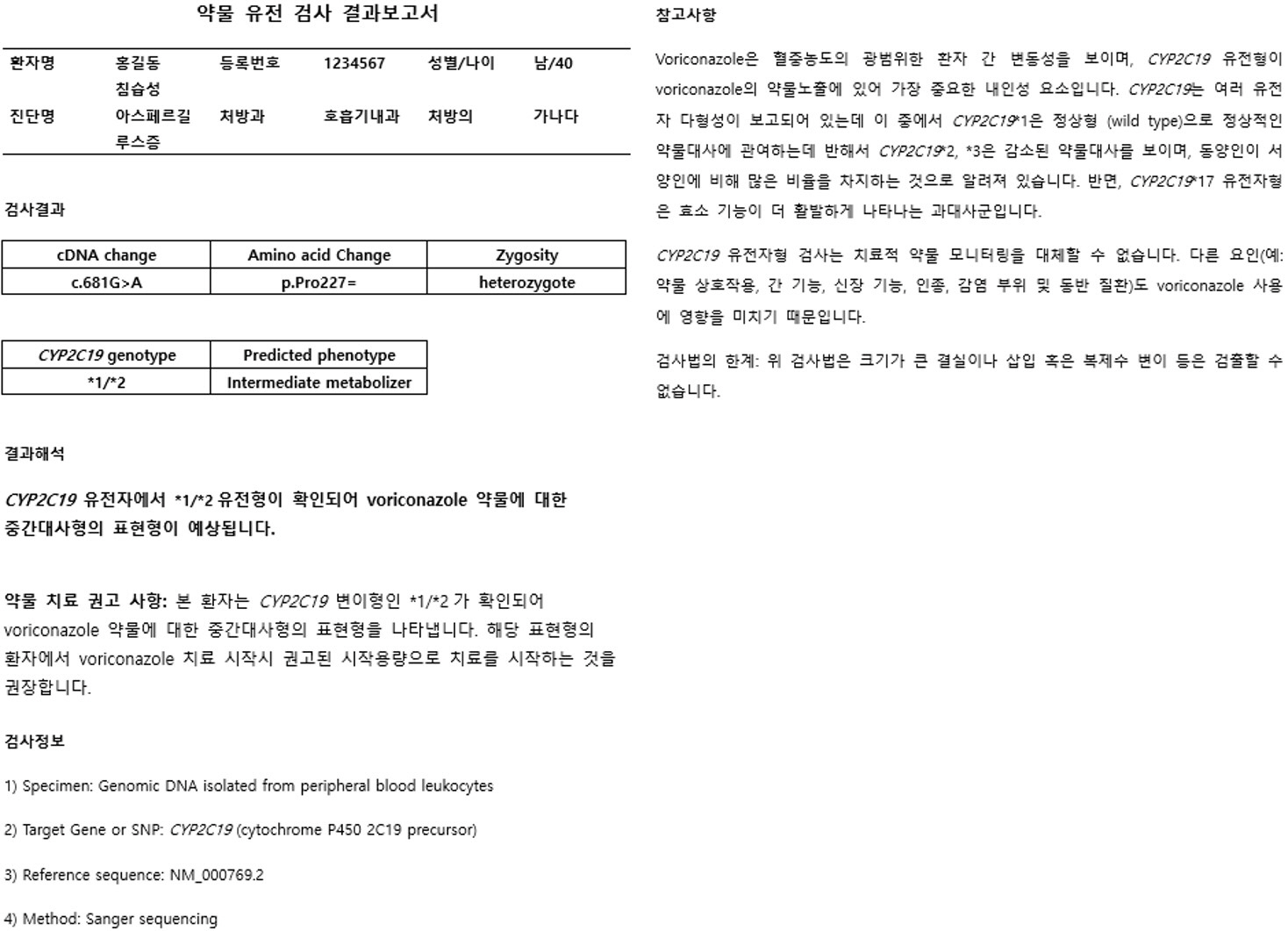
Article14. Lima JJ, Thomas CD, Barbarino J, Desta Z, Van Driest SL, El Rouby N, et al. 2021; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin Pharmacol Ther. 109:1417–23. DOI: 10.1002/cpt.2015. PMID: 32770672. PMCID: PMC7868475.15. Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, et al. 2017; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther. 102:45–51. DOI: 10.1002/cpt.583. PMID: 27981572. PMCID: PMC5474211.
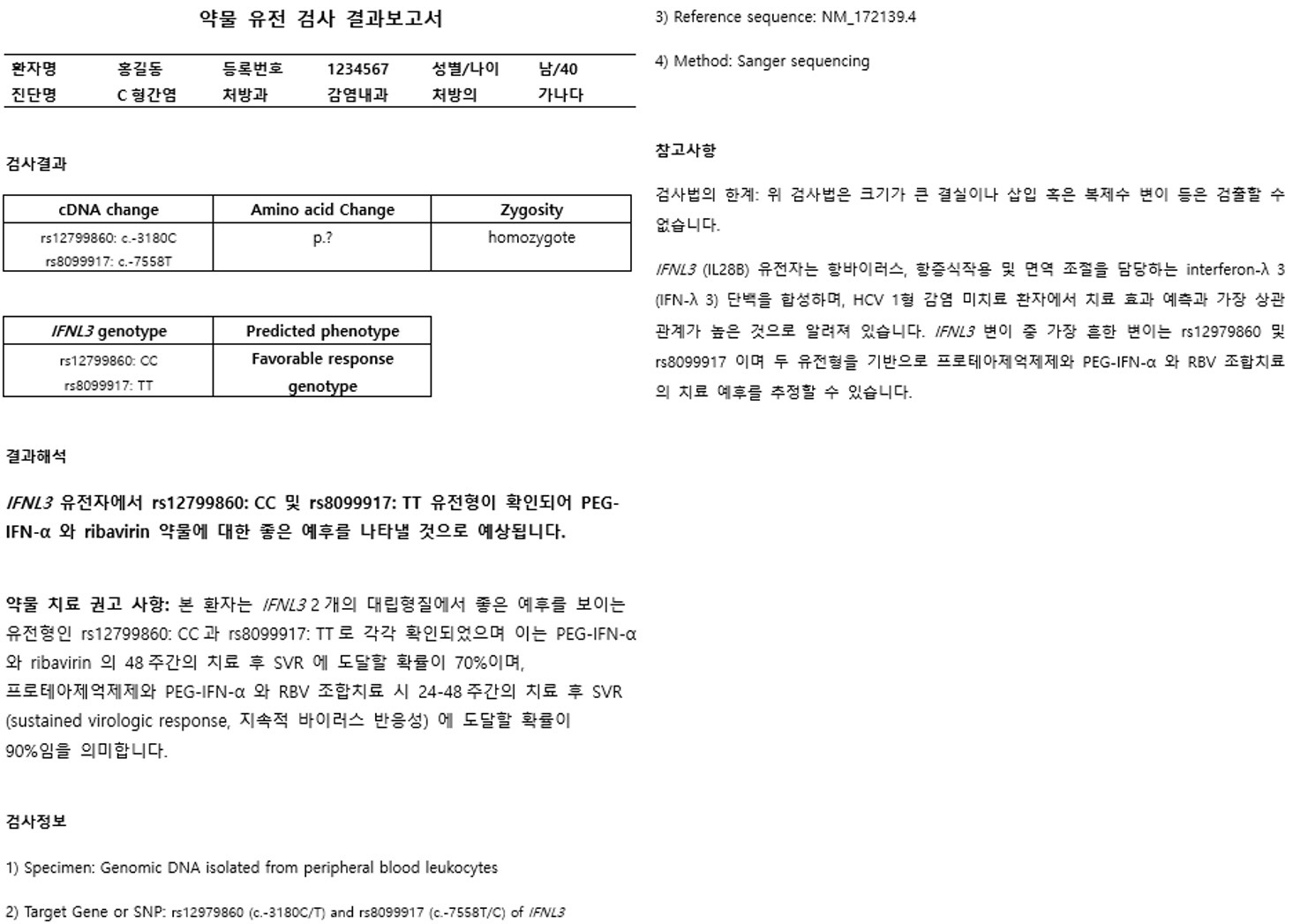
Article16. Muir AJ, Gong L, Johnson SG, Lee MT, Williams MS, Klein TE, et al. 2014; Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for IFNL3 (IL28B) genotype and PEG interferon-α-based regimens. Clin Pharmacol Ther. 95:141–6. DOI: 10.1038/clpt.2013.203. PMID: 24096968. PMCID: PMC3904555.
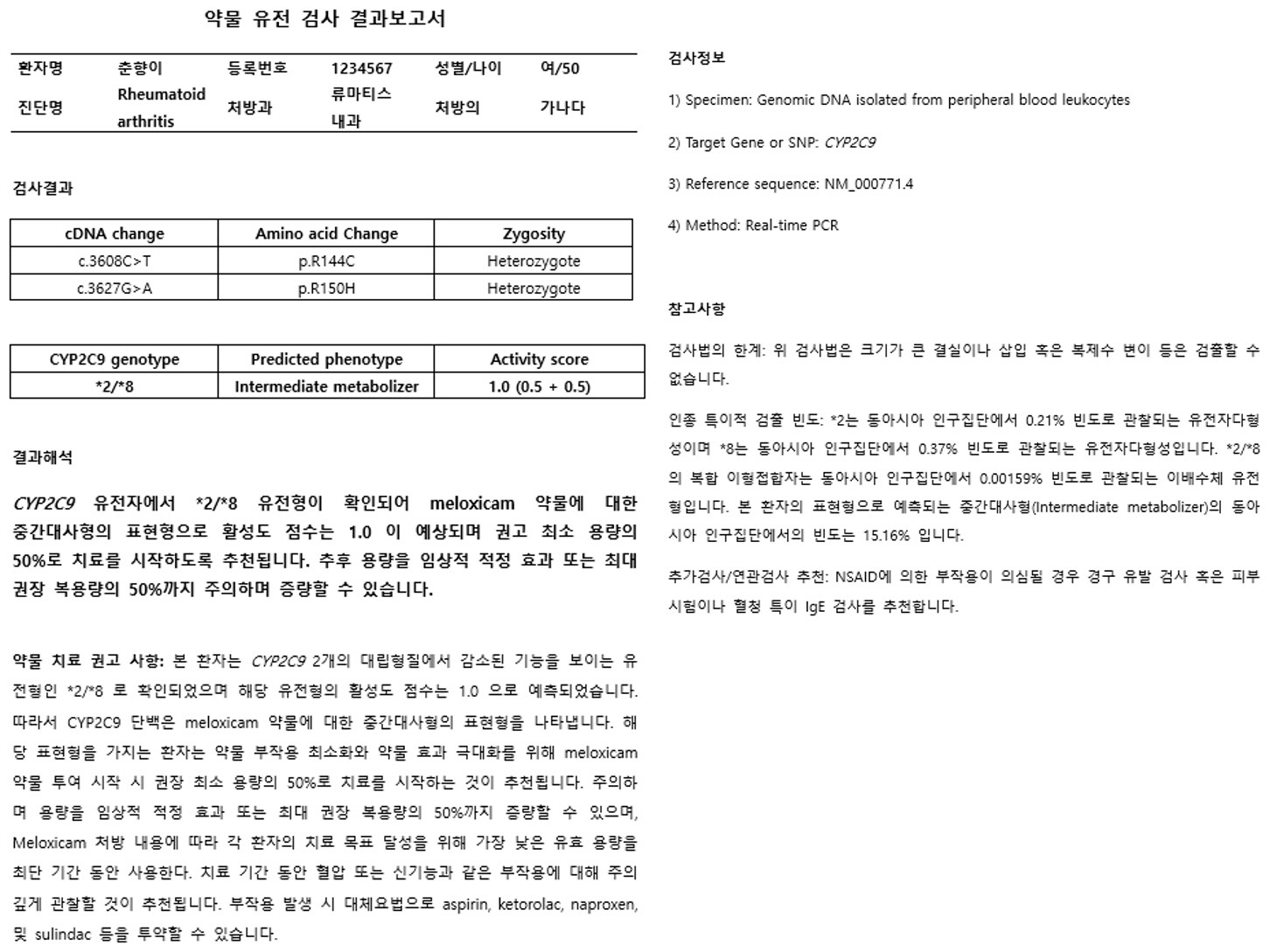
Article17. Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. 2020; Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin Pharmacol Ther. 108:191–200. DOI: 10.1002/cpt.1830. PMID: 32189324. PMCID: PMC8080882.
Article18. Karnes JH, Rettie AE, Somogyi AA, Huddart R, Fohner AE, Formea CM, et al. 2021; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clin Pharmacol Ther. 109:302–9. DOI: 10.1002/cpt.2008. PMID: 32779747. PMCID: PMC7831382.19. Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. 2017; Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin Pharmacol Ther. 102:397–404. DOI: 10.1002/cpt.668. PMID: 28198005. PMCID: PMC5546947.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NUDT15 Genotyping in Thiopurine Drug Therapy
- Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines Part 1
- Recommendations for the Verification of Quantitative Molecular Hemato-Oncology Tests
- Pharmacogenetics in Psychotropic Drugs
- Clinical Pharmacogenetic Testing and Application: Laboratory Medicine Clinical Practice Guidelines