Lab Med Online.
2022 Jul;12(3):209-213. 10.47429/lmo.2022.12.3.209.
A Novel Germline Mutation in DDX41 Predisposed to Myelodysplasia/Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Laboratory Medicine1, Dong-A University College of Medicine, Busan, Korea
- 2Division of Hematology and Oncology, Department of Internal Medicine2, Dong-A University College of Medicine, Busan, Korea
- KMID: 2538608
- DOI: http://doi.org/10.47429/lmo.2022.12.3.209
Abstract
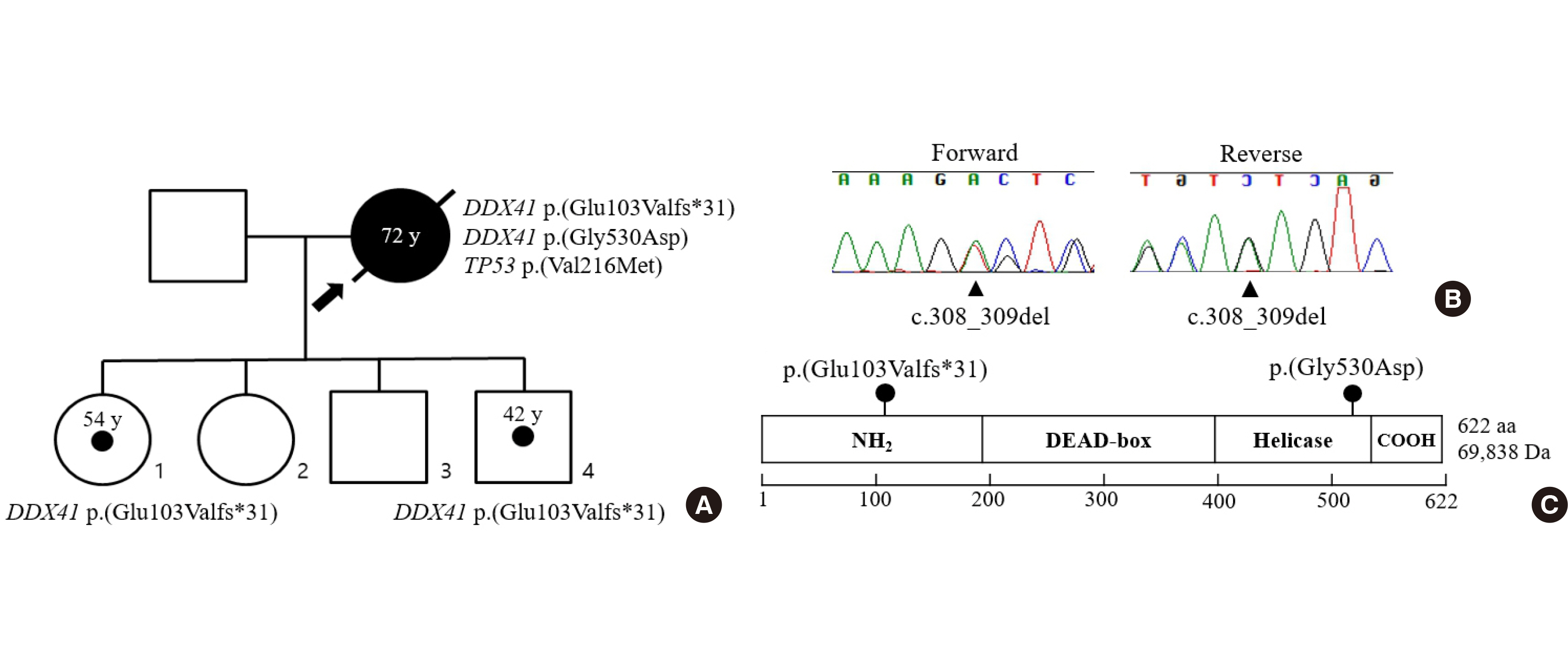
- Germline DDX41lesions indicate a hereditary myelodysplastic syndrome and acute myeloid leukemia (MDS/AML). Canonical somatic mutations in this gene often coincide as a second hit with germline DDX41mutations. We report a patient with inherited MDS/AML containing novel germline DDX41mutations that harbor somatic mutations in the other DDX41allele. The 72-year-old woman was diagnosed with myelodysplastic syndrome with excess blasts-2 (MDS-EB-2) and had gone through 16 rounds of chemotherapy. However, an increase in leukemic myeloblasts was observed in the bone marrow aspiration, resulting in transition to AML. Targeted gene panel sequencing revealed DDX41c.308_309del (p.Glu103Valfs*31) with a variant allele frequency (VAF) of 49%, and DDX41c.1589G>A (p.Gly530Asp) with a VAF of 6%. The c.308_309del variant was confirmed as a germline variant after analyzing buccal DNA. An identical germline DDX41mutation was detected in her unaffected daughter and son. The patient was repeatedly hospitalized for neutropenic fever and eventually expired on account of sepsis. Genetic investigation is crucial for providing appropriate medical management to patients and determining the prognosis of a disease. In addition, it helps to provide appropriate counseling and raise awareness of inherited hematologic malignancies for family members.
Keyword
Figure
Reference
-
1. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.
Article2. West AH, Godley LA, Churpek JE. 2014; Familial myelodysplastic syndrome/acute leukemia syndromes: a review and utility for translational investigations. Ann N Y Acad Sci. 1310:111–8. DOI: 10.1111/nyas.12346. PMID: 24467820. PMCID: PMC3961519.
Article3. Cardoso SR, Ryan G, Walne AJ, Ellison A, Lowe R, Tummala H, et al. 2016; Germline heterozygous DDX41 variants in a subset of familial myelodysplasia and acute myeloid leukemia. Leukemia. 30:2083–6. DOI: 10.1038/leu.2016.124. PMID: 27133828. PMCID: PMC5008455.
Article4. Polprasert C, Schulze I, Sekeres MA, Makishima H, Przychodzen B, Hosono N, et al. 2015; Inherited and somatic defects in DDX41 in myeloid neoplasms. Cancer Cell. 27:658–70. DOI: 10.1016/j.ccell.2015.03.017. PMID: 25920683. PMCID: PMC8713504.
Article5. Linder P. 2006; Dead-box proteins: a family affair-active and passive players in RNP-remodeling. Nucleic Acids Res. 34:4168–80. DOI: 10.1093/nar/gkl468. PMID: 16936318. PMCID: PMC1616962.
Article6. Lewinsohn M, Brown AL, Weinel LM, Phung C, Rafidi G, Lee MK, et al. 2016; Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood. 127:1017–23. DOI: 10.1182/blood-2015-10-676098. PMID: 26712909. PMCID: PMC4968341.7. Polprasert C, Takeda J, Niparuck P, Rattanathammethee T, Pirunsarn A, Suksusut A, et al. 2020; Novel DDX41 variants in Thai patients with myeloid neoplasms. Int J Hematol. 111:241–6. DOI: 10.1007/s12185-019-02770-3. PMID: 31713024.
Article8. Qu S, Li B, Qin T, Xu Z, Pan L, Hu N, et al. 2021; Molecular and clinical features of myeloid neoplasms with somatic DDX41 mutations. Br J Haematol. 192:1006–10. DOI: 10.1111/bjh.16668. PMID: 32307695. PMCID: PMC9205684.
Article9. Sébert M, Passet M, Raimbault A, Rahmé R, Raffoux E, Sicre de Fontbrune F, et al. 2019; Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 134:1441–4. DOI: 10.1182/blood.2019000909. PMID: 31484648.10. Takeda J, Yoshida K, Makishima H, Yoshizato T, Shiozawa Y, Shiraishi Y, et al. 2015; Genetic predispositions to myeloid neoplasms caused by germline DDX41 mutations. Blood. 126:2843. DOI: 10.1182/blood.V126.23.2843.2843.
Article11. Li R, Sobreira N, Witmer PD, Pratz KW, Braunstein EM. 2016; Two novel germline DDX41 mutations in a family with inherited myelodysplasia/acute myeloid leukemia. Haematologica. 101:e228–31. DOI: 10.3324/haematol.2015.139790. PMID: 26944477. PMCID: PMC5013967.12. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. 2012; Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 120:2454–65. DOI: 10.1182/blood-2012-03-420489. PMID: 22740453. PMCID: PMC4425443.
Article13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.
Article14. Cheah JJC, Hahn CN, Hiwase DK, Scott HS, Brown AL. 2017; Myeloid neoplasms with germline DDX41 mutation. Int J Hematol. 106:163–74. DOI: 10.1007/s12185-017-2260-y. PMID: 28547672.15. Jiang Y, Zhu Y, Liu ZJ, Ouyang S. 2017; The emerging roles of the DDX41 protein in immunity and diseases. Protein Cell. 8:83–9. DOI: 10.1007/s13238-016-0303-4. PMID: 27502187. PMCID: PMC5291771.
Article16. Kadono M, Kanai A, Nagamachi A, Shinriki S, Kawata J, Iwato K, et al. 2016; Biological implications of somatic DDX41 p.R525H mutation in acute myeloid leukemia. Exp Hematol. 44:745–754.e4. DOI: 10.1016/j.exphem.2016.04.017. PMID: 27174803.17. Smith ML, Cavenagh JD, Lister TA, Fitzgibbon J. 2004; Mutation of CEBPA in familial acute myeloid leukemia. N Engl J Med. 351:2403–7. DOI: 10.1056/NEJMoa041331. PMID: 15575056.
Article18. Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. 2011; Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 43:1012–7. DOI: 10.1038/ng.913. PMID: 21892162. PMCID: PMC3184204.
Article19. Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, et al. 2002; In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 99:1364–72. DOI: 10.1182/blood.V99.4.1364. PMID: 11830488.20. Kilpivaara O, Mukherjee S, Schram AM, Wadleigh M, Mullally A, Ebert BL, et al. 2009; A germline JAK2 SNP is associated with predisposition to the development of JAK2V617F-positive myeloproliferative neoplasms. Nat Genet. 41:455–9. DOI: 10.1038/ng.342. PMID: 19287384. PMCID: PMC3676425.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Pediatric Case of Acute Myeloid Leukemia with t(3;5)(q25;q34)
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- Myeloid Sarcoma of Peritoneum in Acute Myeloid Leukemia Patient with Inversion of Chromosome 16
- A Case of Myeloid Sarcoma Preceding the Diagnosis of Acute Myeloid Leukemia
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy