Lab Med Online.
2022 Apr;12(2):122-128. 10.47429/lmo.2022.12.2.122.
External Quality Assessment of Serum Protein and Immunofixation Electrophoresis in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2538595
- DOI: http://doi.org/10.47429/lmo.2022.12.2.122
Abstract
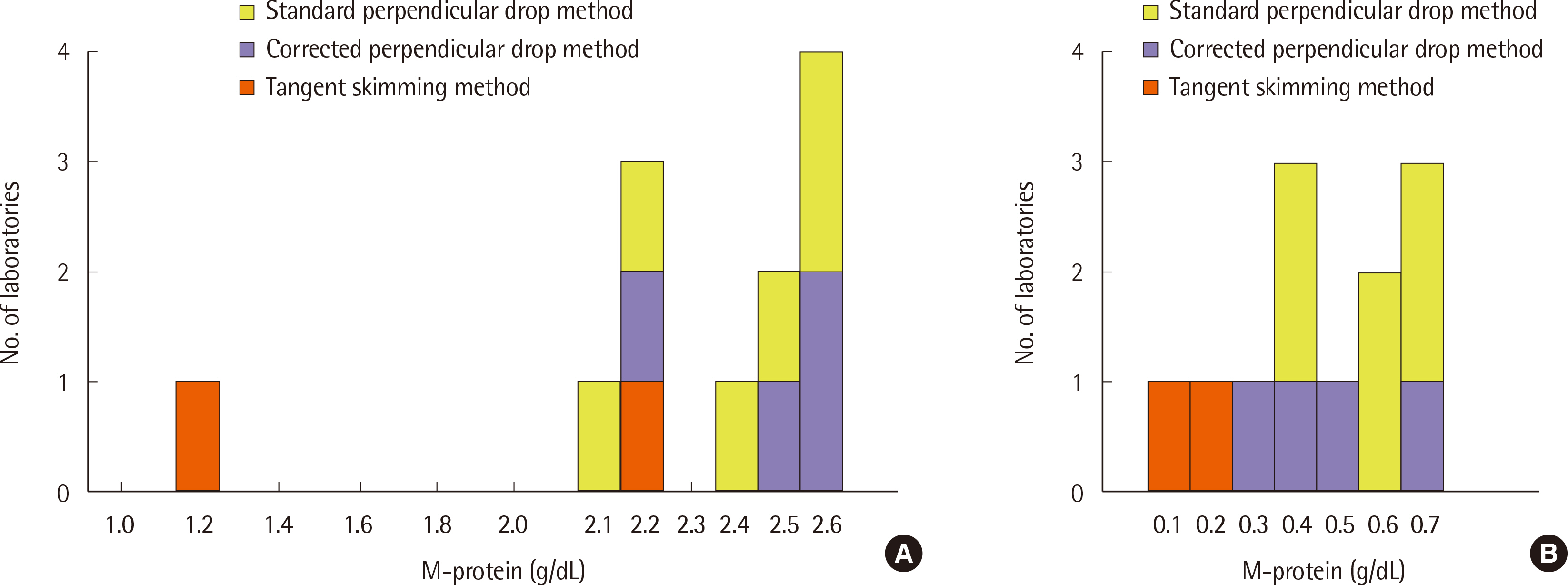
- In Korea, many laboratories rely on inter-laboratory proficiency testing for external quality control of serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE). Therefore, we conducted an external quality assessment in 12 clinical laboratories. Five test samples were prepared by pooling residual samples together in our laboratory according to the SPEP pattern or the existence of monoclonal proteins, and then aliquoting them. Each clinical laboratory carried out SPEP and IFE tests, quantified each fraction and monoclonal protein (if present), and produced the interpretation reports according to the method of each laboratory. Monoclonal protein was detected in two of the five test samples, and the detection of monoclonal proteins and the isotyping results were consistent in all participating institutions. There were no significant variations in the quantities of albumin, alpha-1, and -2 globulin fractions, and the whole beta-gamma region. However, the values of beta-1, -2, and gamma globulin fractions differed significantly, and there was a large variation in the reported monoclonal protein concentrations. The quantitative values obtained using the tangent skimming method were much lower than those obtained using the other methods. In this study, the interpretation reports were generally consistent, but the quantitative values were different between the participating laboratories. This is the first study to approach the external quality assessment of SPEP and IFE in Korea. Further studies are required to establish external quality control management for these tests.
Keyword
Figure
Reference
-
1. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. 2016; International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17:e328–46. DOI: 10.1016/S1470-2045(16)30206-6. PMID: 27511158.
Article2. Durie BGM, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. 2006; International uniform response criteria for multiple myeloma. Leukemia. 20:1467–73. DOI: 10.1038/sj.leu.2404284. PMID: 16855634.
Article3. Azim W, Azim S, Ahmed K, Shafi H, Rafi T, Luqman M. 2004; Diagnostic significance of serum protein electrophoresis. Biomedica. 20:40–4. DOI: 10.29271/jcpsp.2021.07.864,. PMID: 34271795.4. Vavricka SR, Burri E, Beglinger C, Degen L, Manz M. 2009; Serum protein electrophoresis: an underused but very useful test. Digestion. 79:203–10. DOI: 10.1159/000212077. PMID: 19365122.
Article5. O'Connell TX, Horita TJ, Kasravi B. 2005; Understanding and interpreting the serum protein electrophoresis. Am Fam Physician. 71:105–12.6. Weber D, Treon SP, Emmanouilides C, Branagan AR, Byrd JC, Bladé J, et al. 2003; Uniform response criteria in Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 30:127–31. DOI: 10.1053/sonc.2003.50037. PMID: 12720121.
Article7. Booth RA, McCudden CR, Balion CM, Blasutig IM, Bouhtiauy I, Rodriguez-Capote K, et al. 2018; Candidate recommendations for protein electrophoresis reporting from the Canadian Society of Clinical Chemists Monoclonal Gammopathy Working Group. Clin Biochem. 51:10–20. DOI: 10.1016/j.clinbiochem.2017.10.013. PMID: 29061378.
Article8. Moss MA. 2016; Moving towards harmonized reporting of serum and urine protein electrophoresis. Clin Chem Lab Med. 54:973–9. DOI: 10.1515/cclm-2015-0937. PMID: 26824981.
Article9. Tate JR, Smith JD, Wijeratne N, Mollee P. 2019; Proposed addendum to 2012 recommendations for standardised reporting of protein electrophoresis in Australia and New Zealand. Clin Biochem Rev. 40:23–30. PMID: 30828117. PMCID: PMC6370285.10. Tate J, Caldwell G, Daly J, Gillis D, Jenkins M, Jovanovich S, et al. 2012; Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann Clin Biochem. 49:242–56. DOI: 10.1258/acb.2011.011158. PMID: 22402916.
Article11. Cho J, Lee DH, Rim JH, Lee SG, Kim Y, Kim JH. 2022; Current status of serum protein and immunofixation electrophoresis from 29 hospitals in Korea. Lab Med Online. 12:91–9. DOI: 10.47429/lmo.2022.12.2.91.
Article12. de Kat Angelino CM, Jacobs JFM. 2021; External quality assessment of M-protein diagnostics: a realistic impression of the accuracy and precision of M-protein quantification. Clin Chem Lab Med. 59:1063–8. DOI: 10.1515/cclm-2020-1810. PMID: 33544500.
Article13. Turner KA, Frinack JL, Ettore MW, Tate JR, Graziani MS, Jacobs JFM, et al. 2020; An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study. Part I: factors impacting limit of quantitation of serum protein electrophoresis. Clin Chem Lab Med. 58:533–46. DOI: 10.1515/cclm-2019-1104. PMID: 31940284.
Article14. Keren DF, Schroeder L. 2016; Challenges of measuring monoclonal proteins in serum. Clin Chem Lab Med. 54:947–61. DOI: 10.1515/cclm-2015-0862. PMID: 26910744.
Article15. Schild C, Wermuth B, Trapp-Chiappini D, Egger F, Nuoffer JM. 2008; Reliability of M protein quantification: comparison of two peak integration methods on Capillarys 2. Clin Chem Lab Med. 46:876–7. DOI: 10.1515/CCLM.2008.146. PMID: 18601614.
Article16. Wijeratne N, Tate JR, Wienholt L, Mollee P. 2019; Report of the survey conducted by RCPAQAP on current practice for paraprotein and serum free light chain measurement and reporting: a need for harmonisation. Clin Biochem Rev. 40:31–42. PMID: 30828118. PMCID: PMC6370284.17. Willrich MAV, Long TA, Bashleben C, Fink SL, Rudolf JW, Peterson D, et al. 2020; Performance of perpendicular drop versus tangent skimming gating of M-protein in proficiency testing challenges. Clin Chem Lab Med. 59:e19–22. DOI: 10.1515/cclm-2020-0697. PMID: 32628626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- External Quality Assessment and Clinical Laboratory Guidelines for Serum Protein and Immunofixation Electrophoresis in Korea
- Current Status of Serum Protein and Immunofixation Electrophoresis from 29 Hospitals in Korea
- A Case of Multiple Myeloma with Biclonal (IgG-K and IgA-K) M-proteins
- A Case of Multiple Myeloma Showing Marked Differences in Serum IgG Levels between Protein Electrophoresis and Turbidimetry
- Transferrin Analysis by Immunofixation for The Diagnosis of Cerebrospinal Fluid Leakage