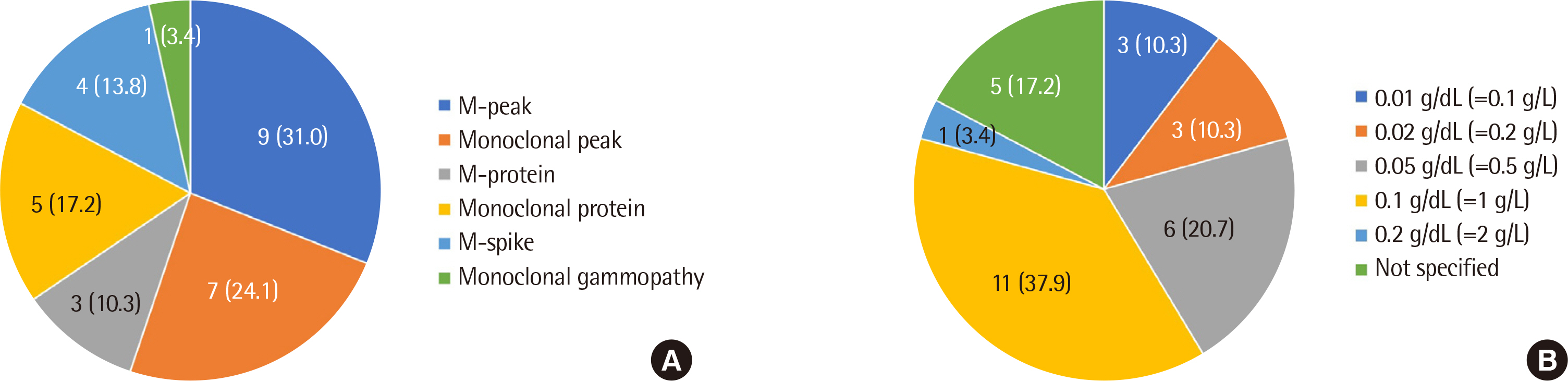Lab Med Online.
2022 Apr;12(2):91-99. 10.47429/lmo.2022.12.2.91.
Current Status of Serum Protein and Immunofixation Electrophoresis from 29 Hospitals in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 4Department of Laboratory Medicine, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- KMID: 2538591
- DOI: http://doi.org/10.47429/lmo.2022.12.2.91
Abstract
- Background
This study aimed to investigate the current status of serum protein electrophoresis (SPEP) and immunofixation electrophoresis (IFE) in Korea.
Methods
Two structured questionnaires were developed using the Naver online platform and provided to clinical pathologists in charge of clinical chemistry. The first questionnaire consisted of 21 questions regarding the methods of testing, quantitation, and interpretation reports. A second questionnaire was administered on the name of the instrument and quality control.
Results
A total of 29 hospital clinical laboratories responded to our first survey, and among them, 15 hospitals participated in the second survey. Twenty-six (89.7%) laboratories reported both quantitative values and interpretation reports, and 24 (82.8%) laboratories presented the ratio of each globulin fraction along with the quantitative values. However, the reporting unit and decimal place, fractionation of each globulin, commentation, terminology, and quantitation method for a monoclonal protein varied among the participating laboratories. Seventeen (58.6%) laboratories performed the inter-laboratory proficiency test, and there were differences in the methods of internal and external quality control among the laboratories.
Conclusions
Variations in SPEP and IFE tests, including quantitation, interpretation reports, and quality control according to the laboratories, were observed. Although this study included a limited number of hospitals, it is the first to report the current status of SPEP and IFE in Korea. Harmonization of methods for reporting results and interpretation reports is needed to reduce the potential inconsistencies in the results.
Keyword
Figure
Cited by 2 articles
-
External Quality Assessment of Serum Protein and Immunofixation Electrophoresis in Korea
Jooyoung Cho, Dong Hyun Lee, John Hoon Rim, Sang-Guk Lee
Lab Med Online. 2022;12(2):122-128. doi: 10.47429/lmo.2022.12.2.122.Performance Evaluation of Capillarys 3 TERA for Serum and Urine Protein Capillary Electrophoresis
Jung-Un Nam, Joonsang Yu, Seunghoo Lee, Sollip Kim, Woochang Lee, Sail Chun
Lab Med Online. 2025;15(2):130-138. doi: 10.47429/lmo.2025.15.2.130.
Reference
-
1. Keren DF. 2012. Protein electrophoresis in clinical diagnosis. London, NW: Hodder Arnold.2. Azim W, Azim S, Ahmed K, Shafi H, Rafi T, Luqman M. 2004; Diagnostic significance of serum protein electrophoresis. Biomedica. 20:40–4. DOI: 10.29271/jcpsp.2021.07.864,. PMID: 34271795.3. Vavricka SR, Burri E, Beglinger C, Degen L, Manz M. 2009; Serum protein electrophoresis: an underused but very useful test. Digestion. 79:203–10. DOI: 10.1159/000212077. PMID: 19365122.
Article4. O'Connell TX, Horita TJ, Kasravi B. 2005; Understanding and interpreting the serum protein electrophoresis. Am Fam Physician. 71:105–12.5. McCudden CR, Mathews SP, Hainsworth SA, Chapman JF, Hammett-Stabler CA, Willis MS, et al. 2008; Performance comparison of capillary and agarose gel electrophoresis for the identification and characterization of monoclonal immunoglobulins. Am J Clin Pathol. 129:451–8. DOI: 10.1309/6KT8N49BRNVVVBT1. PMID: 18285269.
Article6. Tate J, Caldwell G, Daly J, Gillis D, Jenkins M, Jovanovich S, et al. 2012; Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann Clin Biochem. 49:242–56. DOI: 10.1258/acb.2011.011158. PMID: 22402916.
Article7. Tate JR, Smith JD, Wijeratne N, Mollee P. 2019; Proposed addendum to 2012 recommendations for standardised reporting of protein electrophoresis in Australia and New Zealand. Clin Biochem Rev. 40:23–30. PMID: 30828117. PMCID: PMC6370285.8. Booth RA, McCudden CR, Balion CM, Blasutig IM, Bouhtiauy I, Rodriguez-Capote K, et al. 2018; Candidate recommendations for protein electrophoresis reporting from the Canadian Society of Clinical Chemists Mono-clonal Gammopathy Working Group. Clin Biochem. 51:10–20. DOI: 10.1016/j.clinbiochem.2017.10.013. PMID: 29061378.
Article9. Keren DF, Schroeder L. 2016; Challenges of measuring monoclonal proteins in serum. Clin Chem Lab Med. 54:947–61. DOI: 10.1515/cclm-2015-0862. PMID: 26910744.
Article10. Kyle RA. 1994; The monoclonal gammopathies. Clin Chem. 40:2154–61. DOI: 10.1093/clinchem/40.11.2154. PMID: 7955402.
Article11. McCudden CR, Booth RA, Lin DCC, McCurdy A, Rupani N, Kew A. 2018; Synoptic reporting for protein electrophoresis and immunofixation. Clin Biochem. 51:21–8. DOI: 10.1016/j.clinbiochem.2017.09.020. PMID: 28964759.
Article12. Clavijo A, Ryan N, Xu H, Singh G. 2020; Measurement of monoclonal immunoglobulin protein concentration in serum protein electrophoresis: comparison of automated vs manual/human readings. Lab Med. 51:252–8. DOI: 10.1093/labmed/lmz055. PMID: 32374393.
Article13. Bender LM, Cotten SW, Fedoriw Y, Willis MS, McCudden CR. 2013; Evaluation of digital images for identification and characterization of monoclonal immunoglobulins by immunofixation. Clin Biochem. 46:255–8. DOI: 10.1016/j.clinbiochem.2012.10.030. PMID: 23127385.
Article14. Regeniter A, Siede WH. 2018; Peaks and tails: Evaluation of irregularities in capillary serum protein electrophoresis. Clin Biochem. 51:48–55. DOI: 10.1016/j.clinbiochem.2017.09.017. PMID: 28965683.
Article15. Chan PC, Chen Y, Randell EW. 2018; On the path to evidence-based reporting of serum protein electrophoresis patterns in the absence of a discernible monoclonal protein - A critical review of literature and practice suggestions. Clin Biochem. 51:29–37. DOI: 10.1016/j.clinbiochem.2017.09.010. PMID: 28916439.
Article16. Jacobs JFM, Turner KA, Graziani MS, Frinack JL, Ettore MW, Tate JR, et al. 2020; An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study. Part II: limit of detection and follow-up of patients with small M-proteins. Clin Chem Lab Med. 58:547–59. DOI: 10.1515/cclm-2019-1105. PMID: 31940285.
Article17. Wijeratne N, Tate JR, Wienholt L, Mollee P. 2019; Report of the survey conducted by RCPAQAP on current practice for paraprotein and serum free light chain measurement and reporting: a need for harmonisation. Clin Biochem Rev. 40:31–42. PMID: 30828118. PMCID: PMC6370284.18. Schild C, Wermuth B, Trapp-Chiappini D, Egger F, Nuoffer JM. 2008; Reliability of M protein quantification: comparison of two peak integration methods on Capillarys 2. Clin Chem Lab Med. 46:876–7. DOI: 10.1515/CCLM.2008.146. PMID: 18601614.
Article19. Willrich MAV, Long TA, Bashleben C, Fink SL, Rudolf JW, Peterson D, et al. 2020; Performance of perpendicular drop versus tangent skimming gating of M-protein in proficiency testing challenges. Clin Chem Lab Med. 59:e19–22. DOI: 10.1515/cclm-2020-0697. PMID: 32628626.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multiple Myeloma with Biclonal (IgG-K and IgA-K) M-proteins
- External Quality Assessment and Clinical Laboratory Guidelines for Serum Protein and Immunofixation Electrophoresis in Korea
- External Quality Assessment of Serum Protein and Immunofixation Electrophoresis in Korea
- Serum Free Light Chains for Diagnosis and Follow-up of Multiple Myeloma
- A Case of Multiple Myeloma Showing Marked Differences in Serum IgG Levels between Protein Electrophoresis and Turbidimetry