Lab Med Online.
2022 Jan;12(1):33-39. 10.47429/lmo.2022.12.1.33.
Validation of Hematology Rapid Reporting System for Complete Blood Cell Count with Differential
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2538581
- DOI: http://doi.org/10.47429/lmo.2022.12.1.33
Abstract
- Background
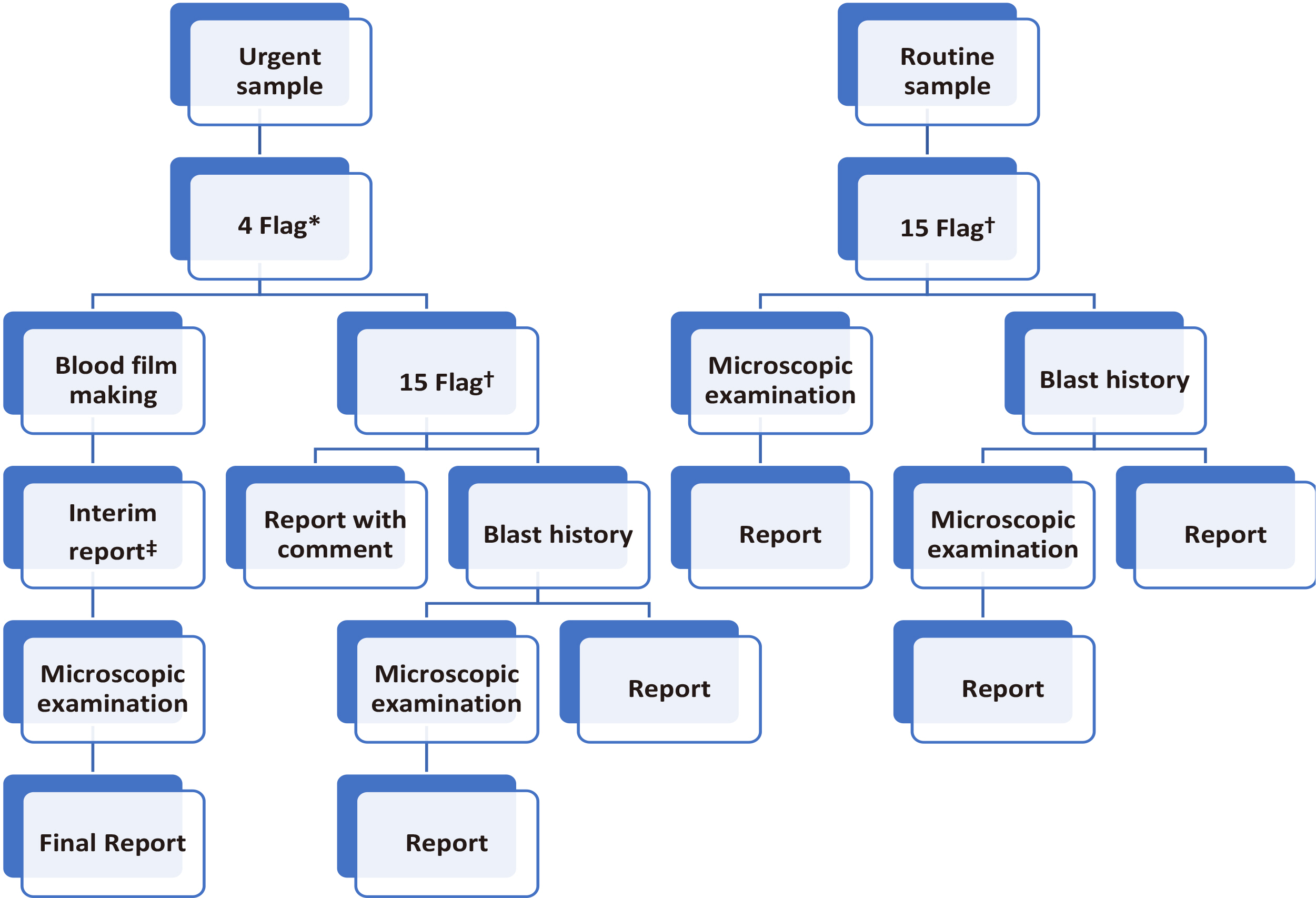
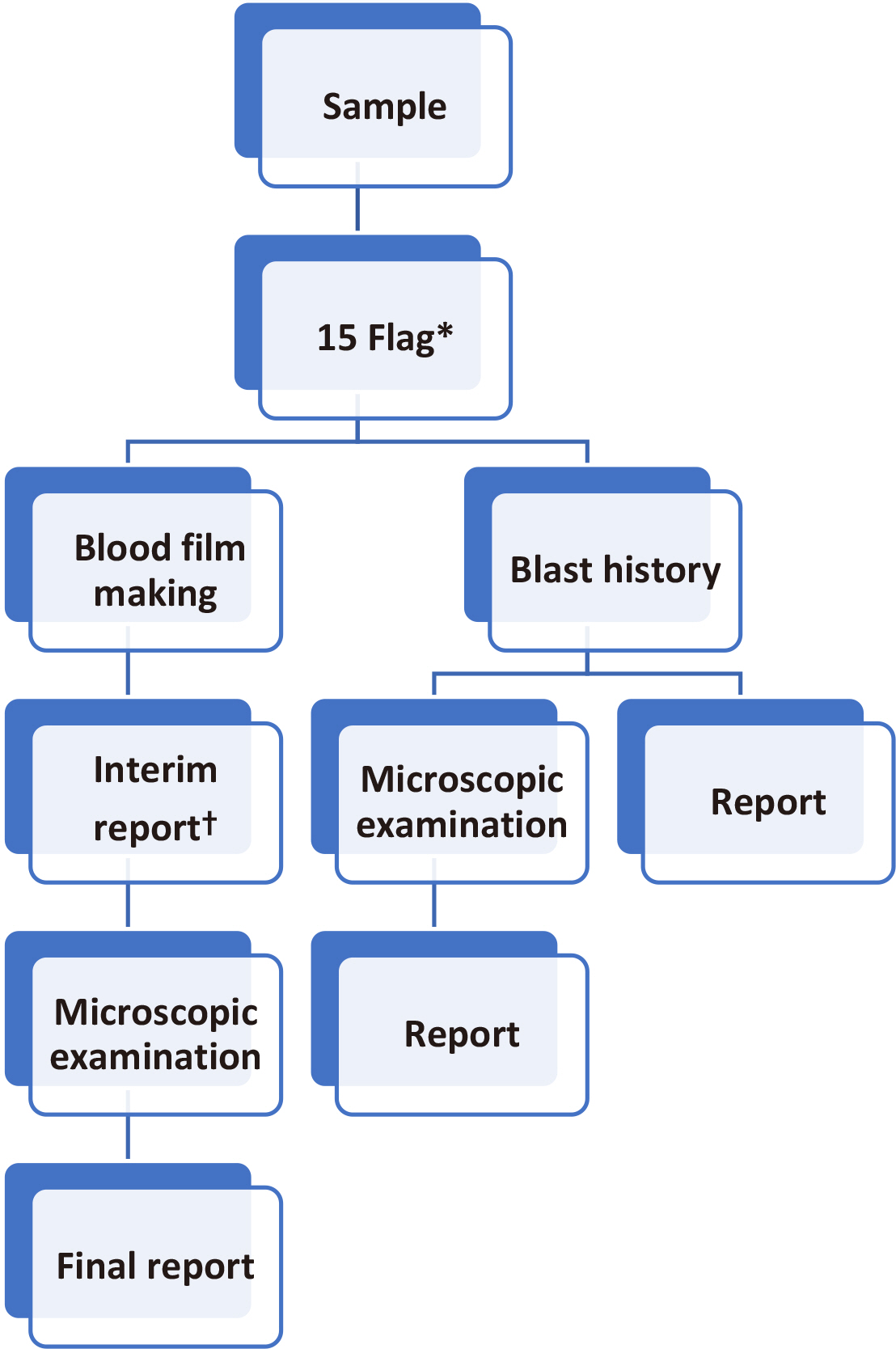
Complete blood cell count (CBC) test, peripheral blood smears (PBS), and automatic hematology analyzers are used to screen patients with high-risk hematology malignancies. In this study, the workflow of urgent and routine samples and methods to improve the detection rate of blasts were evaluated. The purpose of this study was to validate the hematology rapid reporting system (HRS) for CBC and establish a strategy for the effective use of an automated hematology analyzer in clinical laboratories.
Methods
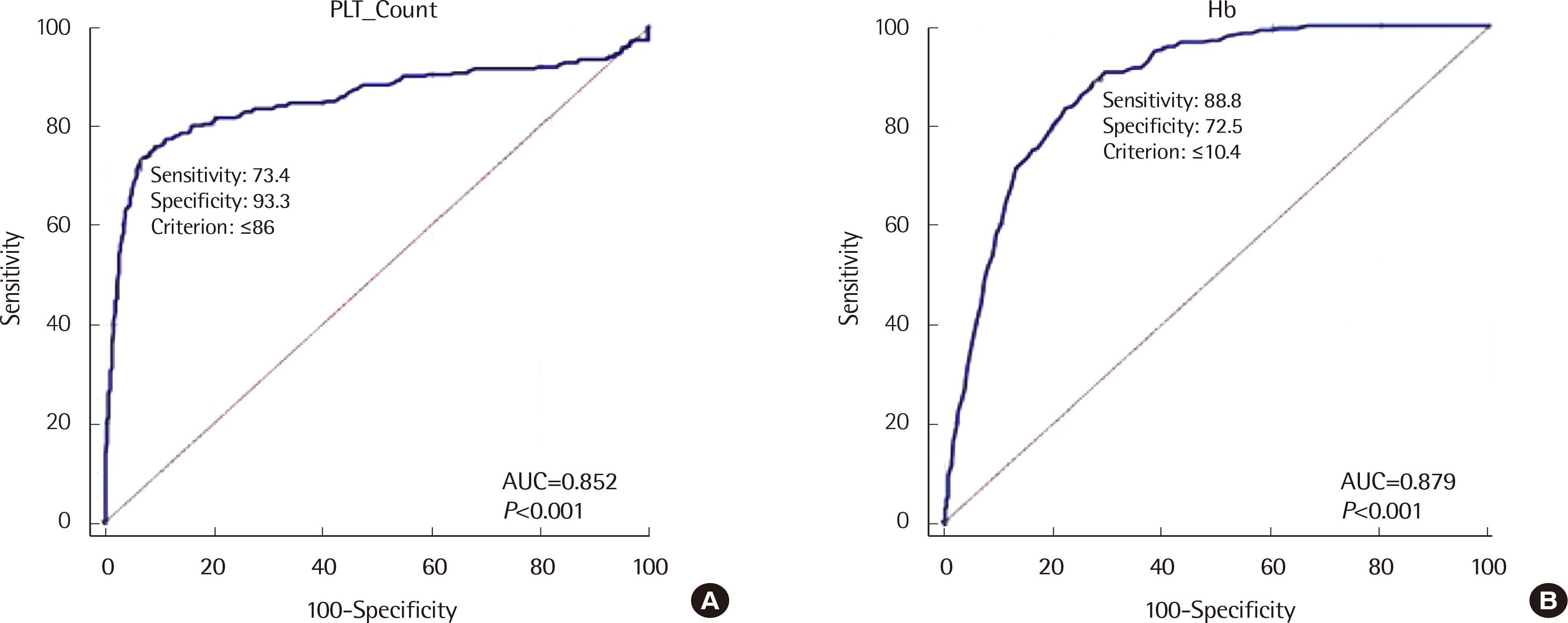
The flag performances of UniCel DxH 800 (Beckman Coulter, USA) and XE-2100 (Sysmex, Japan) systems used for blood tests were analyzed for urgent and routine samples. The results were compared with PBS results. Each test method’s sensitivity and specificity were determined to identify the most efficient blast detection method. In addition, receiver operating characteristic (ROC) curves of various parameters such as CBC and age were analyzed.
Results
The sensitivity and specificity of HRS were 66.80% and 99.85%, respectively, with urgent flags (four flags) and 92.95% and 98.49%, respectively, with routine flags (15 flags). The sensitivity of HRS for routine samples was significantly different from that for urgent samples (66.80% vs. 92.95%; P<0.0001), but no significant difference was observed in the specificity for HRS between routine and urgent samples (99.85% vs. 98.49%; P=1.00).
Conclusions
HRS using flags and blast history can be effectively used in clinical laboratories. HRS can be used as an effective reporting workflow for differential CBC in clinical laboratories.
Figure
Reference
-
1. Jo YA, Kim M, Kim HS, Kang HJ, Lee YK. 2013; Evaluation of the mindray BC-6800 complete blood counts analyzer. Lab Med Online. 3:131–7. DOI: 10.3343/lmo.2013.3.3.131.
Article2. Briggs CJ, Linssen J, Longair I, Machin SJ. 2011; Improved flagging rates on the Sysmex XE-5000 compared with the XE-2100 reduce the number of manual film reviews and increase laboratory productivity. Am J Clin Pathol. 136:309–16. DOI: 10.1309/AJCPDLR4KGKAFW4W. PMID: 21757605.
Article3. Barnes PW, McFadden SL, Machin SJ, Simson E. 2005; The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 11:83–90. DOI: 10.1532/LH96.05019. PMID: 16024331.
Article4. Bain BJ. 2005; Diagnosis from the blood smear. N Engl J Med. 353:498–507. DOI: 10.1056/NEJMra043442. PMID: 16079373.
Article5. Ohsaka A. 2020; Artificial intelligence (AI) and hematological diseases: establishment of a peripheral blood convolutional neural network (CNN)-based digital morphology analysis system. Rinsho Ketsueki. 61:564–9. DOI: 10.11406/rinketsu.61.564. PMID: 32507825.6. Craig FE. 2017; The utility of peripheral blood smear review for identifying specimens for flow cytometric immunophenotyping. Int J Lab Hematol. 39(1S):41–6. DOI: 10.1111/ijlh.12651. PMID: 28447427.
Article7. Rabizadeh E, Pickholtz I, Barak M, Isakov E, Zimra Y, Froom P. 2015; Acute leukemia detection rate by automated blood count parameters and peripheral smear review. Int J Lab Hematol. 37:44–9. DOI: 10.1111/ijlh.12225. PMID: 24702792.
Article8. Houwen B. 2001; The differential cell count. Lab Hematol. 7:89–100.9. Novis DA, Walsh M, Wilkinson D, St. Louis M, Ben-Ezra J. 2006; Laboratory productivity and the rate of manual peripheral blood smear review: a College of American Pathologists Q-Probes study of 95141 complete blood count determinations performed in 263 institutions. Arch Pathol Lab Med. 130:596–601. DOI: 10.5858/2006-130-596-LPATRO. PMID: 16683868.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Annual Report on External Quality Assessment in Hematology in Korea (1996)
- Annual Report on External Quality Assessment in Hematology in Korea (1997)
- Evaluation of CELL-DYN Sapphire Hematology Analyzer
- Purpose and Criteria for Blood Smear Scan, Blood Smear Examination, and Blood Smear Review
- A Clue to Discovering Unstable Hemoglobin Variants via Abnormal WBC Differential Scattergrams Using the Sysmex Automated Hematology Analyzer