Lab Med Online.
2022 Jan;12(1):1-10. 10.47429/lmo.2022.12.1.1.
Development of Cryopreserved Red Blood Cell Panels as Biological Reference Standards for Performance Evaluation of ABO and D Blood Grouping Reagents
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2538577
- DOI: http://doi.org/10.47429/lmo.2022.12.1.1
Abstract
- Background
Accurate blood typing is essential for blood transfusions, and requires the constant evaluation and maintenance of ABO and D blood grouping reagents. In the present study, we developed cryopreserved red blood cell (RBC) panels and evaluated their feasibility as a standard reference material to verify the quality of ABO and D blood grouping reagents in Korea.
Methods
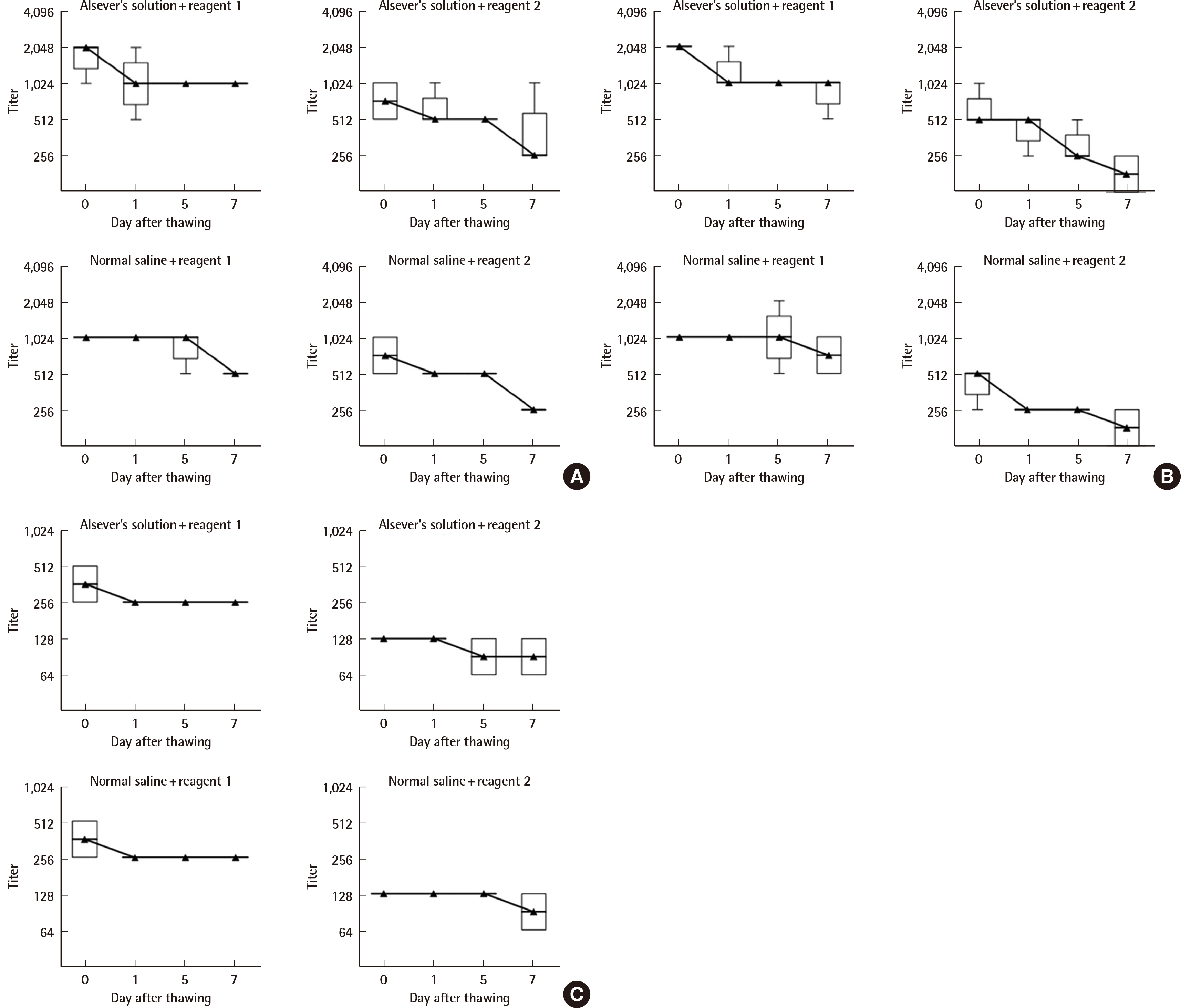
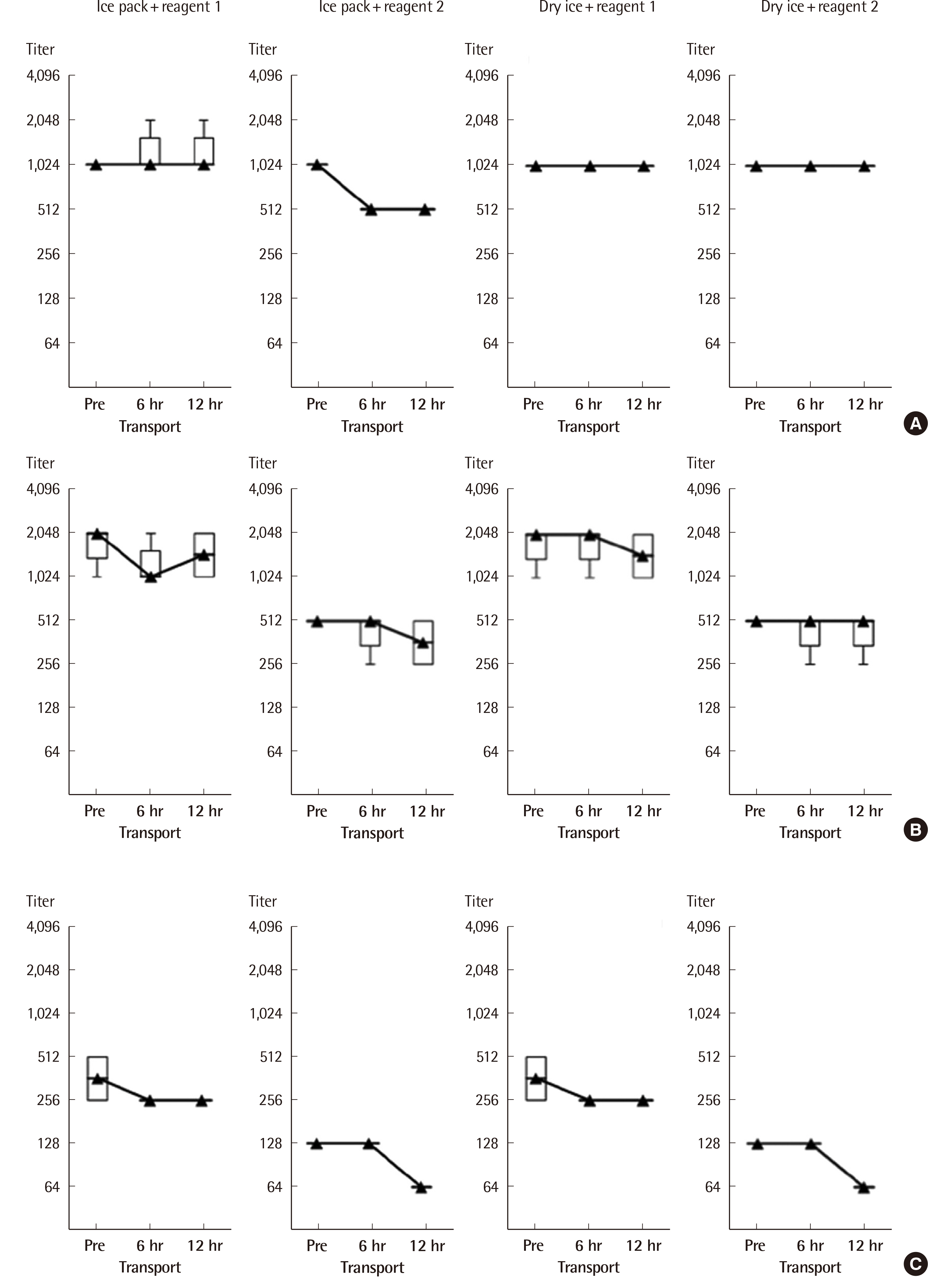
RBC units obtained from healthy donors were cryopreserved using a high-glycerol method. A total of 400 sets of RBC panels were prepared, composed of blood group A (N=5), B (N=5), O (N=10), AB (N=4), Rh D-positive (N=4), Rh D-negative (N=5), and weak-D (N=1), and 200 sets of RBC subgroup panels composed of A2, A2B, A2B3, A1B3, and B3, and A2, A2B, A2B3, A1B3, and A3B (N=1, each). Quality assessment of the cryopreserved RBC panels before and after cryopreservation was performed by measuring their sensitivity, specificity, avidity, and potency titers.
Results
Our cryopreserved ABO and D RBC panels had a sensitivity and specificity of 100% to existing monoclonal blood grouping reagents, regardless of blood type and cryopreservation time. There were no significant differences in the avidity time and potency titers of the cryopreserved RBCs before and after 6 or 12 months of cryopreservation.
Conclusions
The quality parameters measured here suggest that our newly developed cryopreserved RBC panels were reliable for use as a standard reference material for the performance evaluation of anti-A, -B, and -D blood grouping reagents.
Figure
Cited by 1 articles
-
Evaluating the TaqMan Jra-Genotyping Method for Rapidly Predicting the Presence of Anti-Jra Antibodies
Yu-Kyung Koo, Soon Sung Kwon, Eun Jung Suh, Na Hyeong Kim, Hyun Kyung Kim, Youn Keong Cho, Seung Jun Choi, Sinyoung Kim, Kyung-A Lee
Ann Lab Med. 2024;44(5):418-425. doi: 10.3343/alm.2023.0325.
Reference
-
1. Mujahid A, Dickert FL. 2015; Blood group typing: from classical strategies to the application of synthetic antibodies generated by molecular imprinting. Sensors (Basel). 16:51. DOI: 10.3390/s16010051. PMID: 26729127. PMCID: PMC4732084.
Article2. Anstee DJ. 2009; Red cell genotyping and the future of pretransfusion testing. Blood. 114:248–56. DOI: 10.1182/blood-2008-11-146860. PMID: 19411635.
Article3. Kim S, Song S, Kim HO. 2018; Cryopreservation of genotyped ABO subgroup RBCs for quality assurance of ABO grouping reagents. Ann Clin Lab Sci. 48:216–22. PMID: 29678850.4. Lim HH, Kim KH, An GD, Jeong IH, Son YK. 2018; Acute hemolytic transfusion reaction due to ABO-incompatible blood transfusion: a fatal case report and review of the literature. Korean J Blood Transfus. 29:73–8. DOI: 10.17945/kjbt.2018.29.1.73.
Article5. U.S. Department of Health and Human Services Food and Drug Administrations. CFR - Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=660&showFR=1&subpartNode=21:7.0.1.1.8.3. Updated on Oct 01, 2021; Last accessed on Nov 15, 2021.6. Eur-Lex. 2009/886/EC: Commission Decision of 27 November 2009 amending Decision 2002/364/EC on common technical specifications for in vitro diagnostic medical devices. OJ L 318, 4.12.2009, Nov. 27 2009s. https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32009D0886. updated on Nov. 2009.7. India National Institute of Biologicals. 2012. Guidance Manual-Quality control of ABO and Rh blood grouping reagents. Document ID No. NIB/BRL /GM/01. NOIDA. India Ministry of Health and Family Welfare;NOIDA:8. U.S. Department of Health and Human Services Food and Drug Administrations. Overview of IVD regulation. https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation. Updated on Oct 18, 2021; Last accessed on Nov 15, 2021.9. Korea National Institute of Food, Drug Safety Evaluation. Review and approval guidlines for ABO blood grouping reagents in Korea. https://www.nifds.go.kr/brd/m_15/down.do?brd_id=167&seq=7455&data_tp=A&file_seq=1. Updated on Dec 2015.10. U.S. Department of Health and Human Services Food and Drug Administrations. Recommended methods for evaluating potency, specificity, and reactivity of anti-human globulin. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/Draft-Recommended-Methods-for-Evaluating-Potency--Specificity--and-Reactivity-of-Anti-Human-Globulin.pdf. Updated on Mar 1992.11. Lee JY, Oh DJ, park YM. 2010; The frequency and distribution of the ABO subgroups in Korean blood donors. Korean J Blood Transfus. 21:223–9.12. Yang HS, Chun S, Lee SA, Kwon JR, Choi YS, Kim JN, et al. 2017; Transfusion strategy of RhD-negative/variant patients in the Korean population. Lab Med Online. 7:89–93. DOI: 10.3343/lmo.2017.7.3.89.
Article13. Song S, Choi J, Kim S, Kim HO, Min H, Kim J, et al. 2009; Development of cryopreserved red blood cell panels for verifying ABO and D blood grouping reagents. Korean J Blood Transfus. 20:46–54.14. Meryman HT, Hornblower M. 1972; A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion. 12:145–56. DOI: 10.1111/j.1537-2995.1972.tb00001.x. PMID: 5026166.
Article15. Högman CF, Hornblower ML, Flodin M, Gillberg G, Meryman HT, Säfwenberg J. 1986; A simple method for high-quality frozen red cells in blood group serology. Transfusion. 26:434–6. DOI: 10.1046/j.1537-2995.1986.26587020120.x. PMID: 3765037.
Article16. Lelkens CC, Noorman F, Koning JG, Truijens-de Lange R, Stekkinger PS, Bakker JC, et al. 2003; Stability after thawing of RBCs frozen with the high- and low-glycerol method. Transfusion. 43:157–64. DOI: 10.1046/j.1537-2995.2003.00293.x. PMID: 12559010.
Article17. Langston MM, Procter JL, Cipolone KM, Stroncek DF. 1999; Evaluation of the gel system for ABO grouping and D typing. Transfusion. 39:300–5. DOI: 10.1046/j.1537-2995.1999.39399219288.x. PMID: 10204594.
Article18. Gorakshakar A, Gogri H, Ghosh K. 2017; Evolution of technology for molecular genotyping in blood group systems. Indian J Med Res. 146:305–15. DOI: 10.4103/ijmr.IJMR_914_16. PMID: 29355136. PMCID: PMC5793464.19. Quill E. 2008; Medicine. Blood-matching goes genetic. Science. 319:1478–9. DOI: 10.1126/science.319.5869.1478. PMID: 18339916.20. Smith AU. 1950; Prevention of haemolysis during freezing and thawing of red blood-cells. Lancet. 2:910–1. DOI: 10.1016/S0140-6736(50)91861-7. PMID: 14795743.21. Lee E, Sivalingam J, Lim ZR, Chia G, Shi LG, Roberts M, et al. 2018; Review: in vitro generation of red blood cells for transfusion medicine: progress, prospects and challenges. Biotechnol Adv. 36:2118–28. DOI: 10.1016/j.biotechadv.2018.09.006. PMID: 30273713.
Article22. Kim HO. International collaboratory study for performance evaluation criteria of blood grouping reagents and standardization of reagent red cell. https://scienceon.kisti.re.kr/commons/util/originalView.do?cn=TRKO200800006397&dbt=TRKO&rn=. Updated on Apr 2008.23. Rudmann SV. 2005. Blood component preservation and storage. Textbook of blood banking and transfusion medicine. 2nd ed. Philladelpia, PA: Elsevier Health Sciences. p. 274–5.24. Valeri CR, Pivacek LE, Gray AD, Cassidy GP, Leavy ME, Dennis RC, et al. 1989; The safety and therapeutic effectiveness of human red cells stored at -80 degrees C for as long as 21 years. Transfusion. 29:429–37. DOI: 10.1046/j.1537-2995.1989.29589284145.x. PMID: 2734823.25. Valeri CR, Ragno G, Pivacek LE, Cassidy GP, ey R Sr, Hansson-Wicher M, et al. 2000; An experiment with glycerol-frozen red blood cells stored at -80 degrees C for up to 37 years. Vox Sang. 79:168–74. DOI: 10.1046/j.1423-0410.2000.7930168.x. PMID: 11111236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Cryopreserved Red Blood Cell Panels for Verifying ABO and D Blood Grouping Reagents
- Evaluation of DiaCell ABO Red Blood Cell Reagents as a Reverse Typing for ABO Blood Group
- Report on External Proficiency Testing for the ABO and D Blood Group Typing Tests in Blood Centers (2014)
- Report on External Proficiency Testing for Blood Grouping Tests in Blood Centers (2011)
- ABO Genotyping of a Neonate with Mixed Field Agglutination