Cancer Res Treat.
2023 Jan;55(1):245-257. 10.4143/crt.2022.232.
Effect of BRCA1/2 Mutational Status on Survival Outcomes According to Secondary Cytoreductive Surgery and Maintenance Therapy in Platinum-Sensitive Relapsed Ovarian Cancer: A Real-World Evidence Study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea
- KMID: 2538008
- DOI: http://doi.org/10.4143/crt.2022.232
Abstract
- Purpose
This study aimed to investigate the impact of BRCA1/2 mutational status on survival outcomes in patients with platinum-sensitive relapsed (PSR) epithelial ovarian cancer (EOC).
Materials and Methods
We retrospectively identified patients who received secondary treatment for PSR EOC at our institution between January 2007 and June 2021 and who underwent BRCA1/2 gene testing by either germline or somatic methods. The association between BRCA1/2 mutational status and survival outcomes was evaluated. Both secondary cytoreductive surgery (CRS) and maintenance therapy were stratified considering real-world clinical practice.
Results
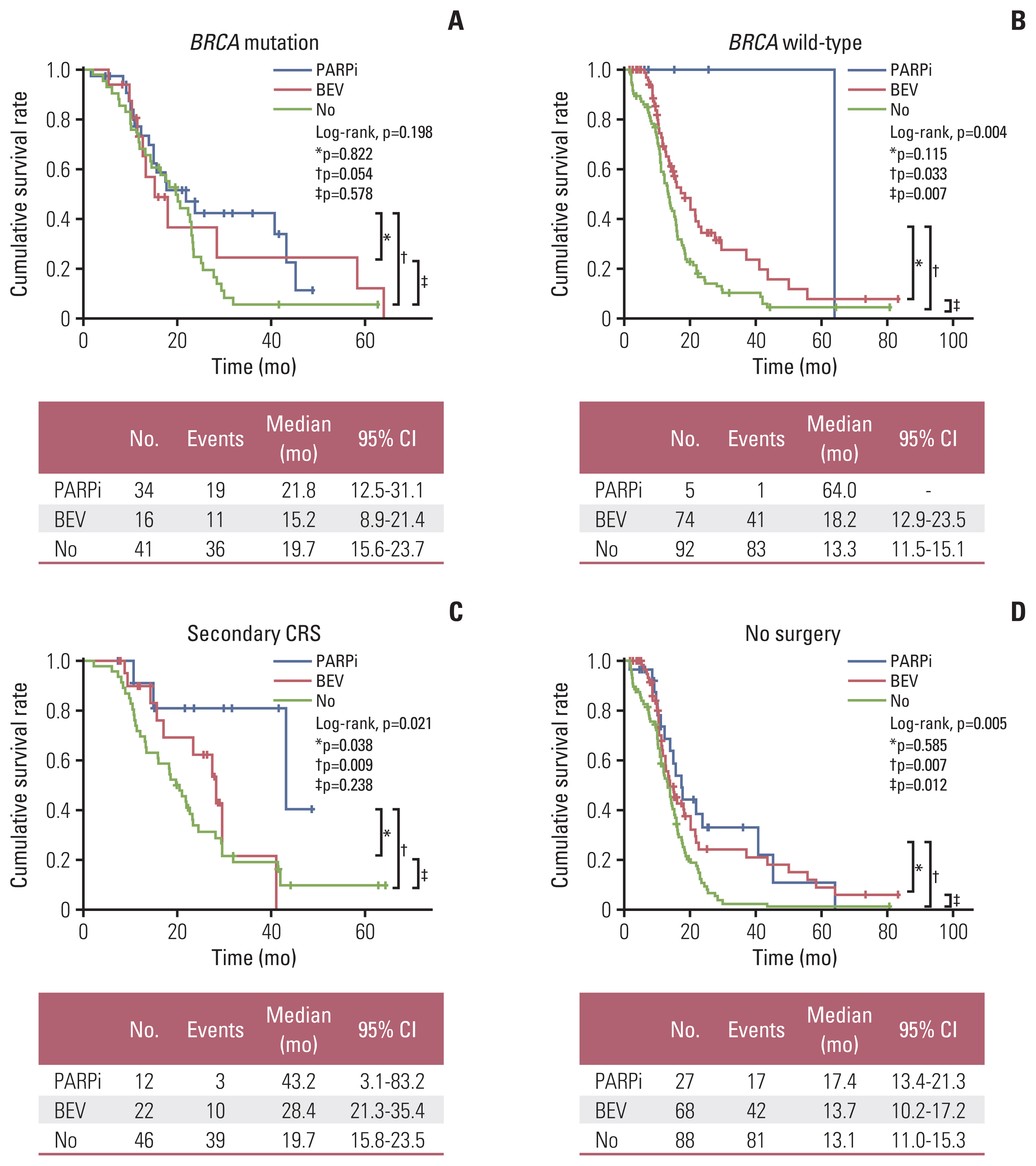
Of 262 patients, 91 (34.7%) and 171 (65.3%) were assigned to BRCA1/2 mutation and wild-type groups, respectively. The two groups had similar proportions of patients undergoing secondary CRS (26.4% vs. 32.7%, p=0.286) and maintenance therapy (54.9% vs. 46.2%, p=0.178). Overall, no differences in progression-free survival (PFS; median, 19.7 vs. 15.1 months, p=0.120) and overall survival (OS; p=0.400) were observed between the two groups. In multivariate analyses, BRCA1/2 mutational status was not associated with PFS (adjusted hazard ratio, 0.816; 95% confidence interval, 0.596 to 1.119; p=0.207). BRCA1/2 mutational status did not affect PFS among patients who underwent secondary CRS (n=80) and among those who did not (n=182) (p=0.074 and p=0.222, respectively). PFS did not differ in the BRCA1/2 mutational status among the patients who received bevacizumab maintenance (n=90, p=0.992).
Conclusion
In this real-world evidence study, BRCA1/2 mutational status itself was not associated with PFS and OS in PSR EOC, which was consistent with whether secondary CRS or not and with bevacizumab maintenance.
Keyword
Figure
Reference
-
References
1. Jung KW, Won YJ, Kang MJ, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res Treat. 2022; 54:345–51.2. Lim MC, Won YJ, Ko MJ, Kim M, Shim SH, Suh DH, et al. Inci-dence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol. 2019; 30:e38.3. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019; 393:1240–53.4. Kim S, Han Y, Kim SI, Kim HS, Kim SJ, Song YS. Tumor evolution and chemoresistance in ovarian cancer. NPJ Precis Oncol. 2018; 2:20.5. National Comprehensive Cancer Network. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1, 2022 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2022. [cited 2022 May 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf .6. Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann Oncol. 2019; 30:672–705.7. Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012; 366:1382–92.8. Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016; 375:2154–64.9. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017; 18:1274–84.10. Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021; 22:620–31.11. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017; 317:2402–16.12. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008; 26:5530–6.13. Kim SI, Lee M, Kim HS, Chung HH, Kim JW, Park NH, et al. Germline and somatic BRCA1/2 gene mutational status and clinical outcomes in epithelial peritoneal, ovarian, and fallopian tube cancer: over a decade of experience in a single institution in Korea. Cancer Res Treat. 2020; 52:1229–41.14. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012; 30:2039–45.15. Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017; 18:779–91.16. Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021; 385:2123–31.17. Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021; 22:439–49.18. Kim SI, Kim JW. Role of surgery and hyperthermic intraperitoneal chemotherapy in ovarian cancer. ESMO Open. 2021; 6:100149.19. Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011; 21:289–95.20. Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019; 381:1929–39.21. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–24.22. Suh DH, Chang SJ, Song T, Lee S, Kang WD, Lee SJ, et al. Practice guidelines for management of ovarian cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol. 2018; 29:e56.23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.24. Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, et al. Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer. 2012; 118:3703–9.25. Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012; 30:2654–63.26. Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM, Brady MF, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019; 37:2317–28.27. Lorusso D, Marchetti C, Conte C, Giudice E, Bolomini G, Vertechy L, et al. Bevacizumab as maintenance treatment in BRCA mutated patients with advanced ovarian cancer: a large, retrospective, multicenter case-control study. Gynecol Oncol. 2020; 159:95–100.28. Vencken P, Kriege M, Hoogwerf D, Beugelink S, van der Burg MEL, Hooning MJ, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011; 22:1346–52.29. Fuso Nerini I, Cesca M, Bizzaro F, Giavazzi R. Combination therapy in cancer: effects of angiogenesis inhibitors on drug pharmacokinetics and pharmacodynamics. Chin J Cancer. 2016; 35:61.30. Baek MH, Park EY, Ha HI, Park SY, Lim MC, Fotopoulou C, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer: a meta-analysis. J Clin Oncol. 2022; 40:1659–70.31. Lee YY, Choi MC, Park JY, Suh DH, Kim JW. Major clinical research advances in gynecologic cancer in 2020. J Gynecol Oncol. 2021; 32:e53.32. Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017; 23:1124–34.33. Marchetti C, Rosati A, Scaletta G, Pietragalla A, Arcieri M, Ergasti R, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer before olaparib maintenance: Still getting any benefit? A case-control study. Gynecol Oncol. 2019; 155:400–5.34. Ha HI, Park EY, Eoh KJ, Lee YJ, Seo SS, Kang S, et al. Clinical outcomes of BRCA1/2 pathogenic variants in ovarian cancer cluster region in patients with primary peritoneal, epithelial ovarian, and fallopian tube cancer. Gynecol Oncol. 2022; 164:415–20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and impact of PARPi monotherapy to subsequent platinum-based chemotherapy in BRCA1/2 mutant ovarian cancer patients with secondary platinum-sensitive relapse
- Prevalence and oncologic outcomes of BRCA1/2 mutation and variant of unknown significance in epithelial ovarian carcinoma patients in Korea
- A phase II trial of cytoreductive surgery combined with niraparib maintenance in platinum-sensitive, secondary recurrent ovarian cancer: SGOG SOC-3 study
- An overview of the current debate between using minimally invasive surgery versus laparotomy for interval cytoreductive surgery in epithelial ovarian cancer
- Secondary acute myeloid leukemia after platinum based chemotherapy for ovarian cancer